Figures & data
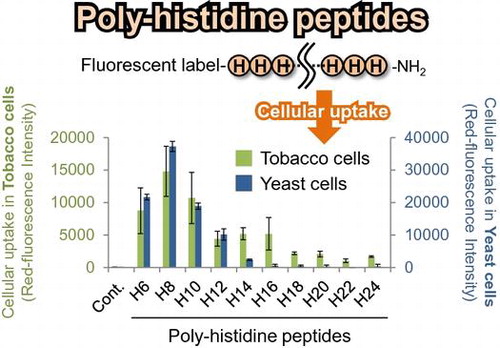
Table 1. Sequence and net charge of the PHPs used this study.
Fig. 1. Cellular uptake of PHPs into BY-2 plant cells.
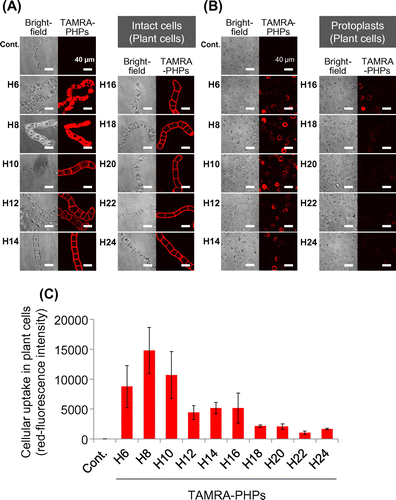
Fig. 2. Cellular uptake of PHPs into NMY51 yeast cells.
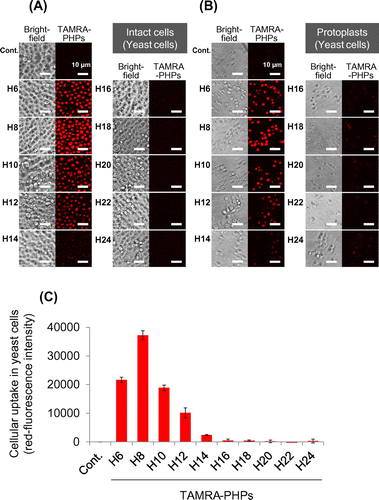
Fig. 3. Distribution and cellular uptake route of PHPs in the BY-2 plant cells.
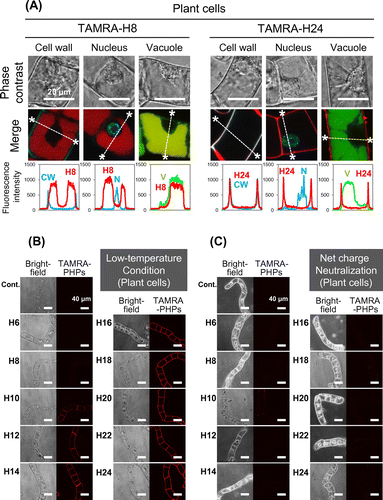
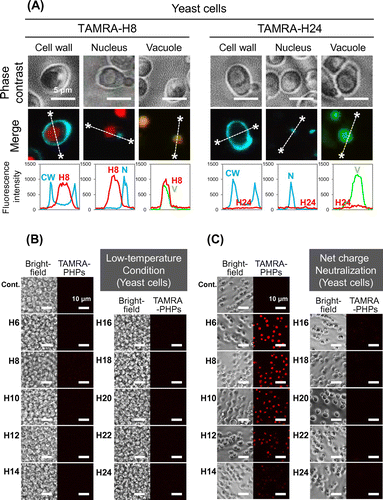
Fig. 4. Distribution and cellular uptake route of PHPs in the NMY51 yeast cells.