Figures & data
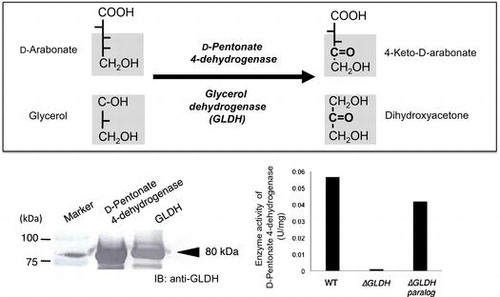
Fig. 1. Effects of EDTA on D-pentonate 4-dehydrogenase.
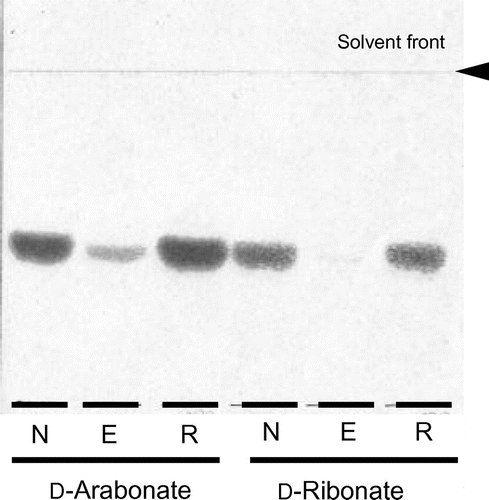
Fig. 2. Effects of EDTA on D-fructose 5-dehydrogenase.
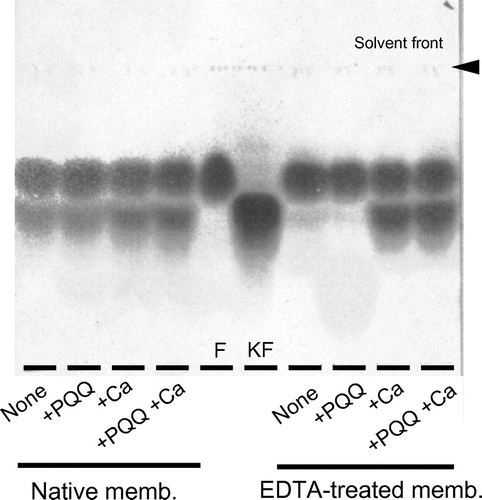
Fig. 3. CM-Toyopearl chromatography of D-pentonate 4-dehydrogenase.
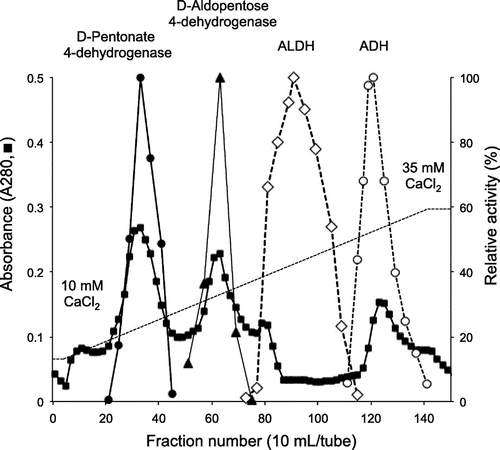
Fig. 4. Gel filtration, absorption spectrum (A) and SDS-PAGE (B) of purified D-pentonate 4-dehydrogenase.
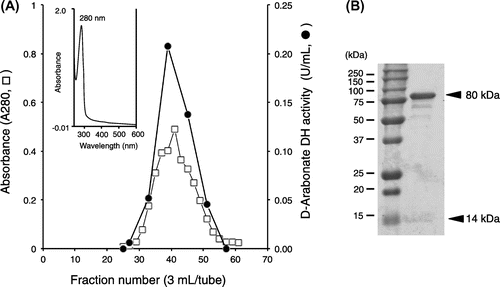
Fig. 5. Reaction versatility of GLDH to D-pentonate, D-fructose, D-psicose, and D-erythronate on the basis of the Bertrand–Hudson’s rule.
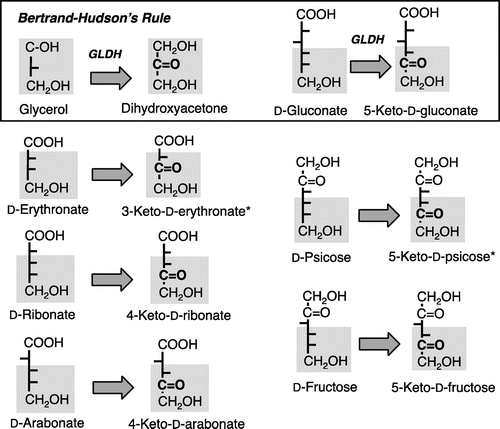
Fig. 6. Immunoblotting analysis of D-pentonate 4-dehydrogenase and glycerol dehydrogenase (GLDH).
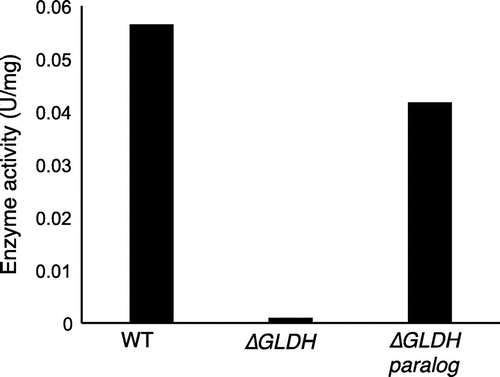
Fig. 7. Effect of gene disruption on D-pentonate 4-dehydrogenase activity.