Figures & data
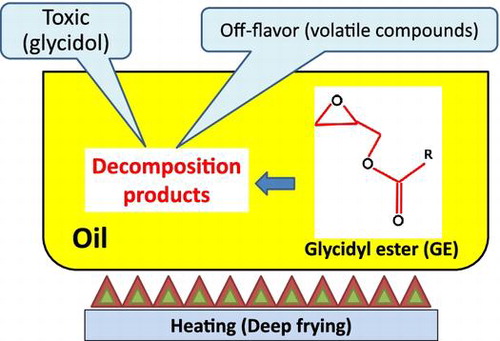
Fig. 1. Changes in the weight of glycidyl palmitate (GP) and tripalmitin (TP) during heating at 180 and 200 °C. ● GP (200 °C), ▴ GP (180 °C), ■ TP (200 °C), and ♦ TP (180 °C). Data show the mean and standard deviation of triplicate.
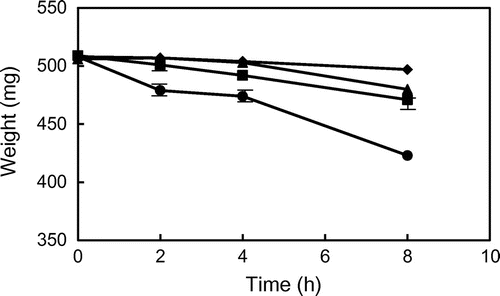
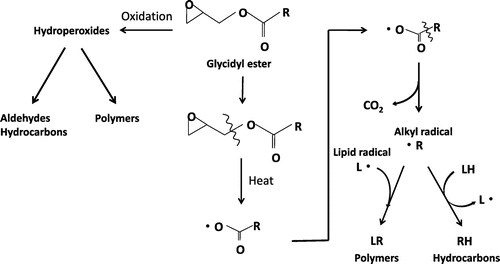
Fig. 2. TLC-FID chromatograms of GP and TP after heating at 180 °C. A TP (2 h), B TP (4 h), C TP (8 h), D GP (2 h), E GP (4 h), and F GP (8 h). GP, glycidyl palmitate; TP, tripalmitin; PC, polar compounds.
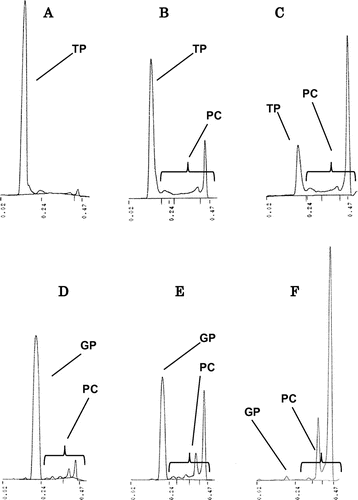
Fig. 3. Changes in the relative ratio of GP, TP, and polar compounds (PC) to total peak areas during heating at 180 and 200 °C. ● GP (200 °C), ▴ GP (180 °C), ■ TP (200 °C), and ♦ TP (180 °C). Data shows the mean and standard deviation of triplicate.
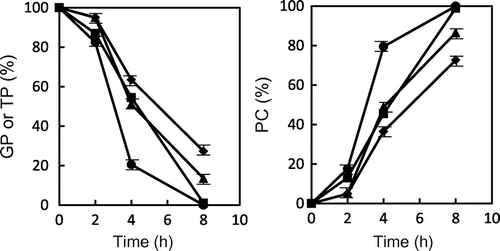
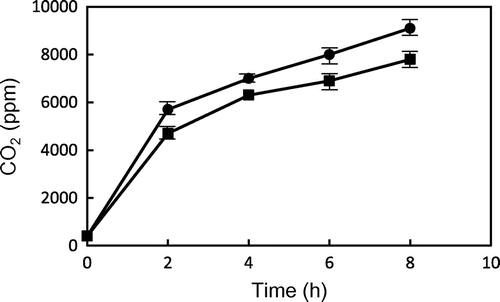
Fig. 4. Gas chromatograms of head space gas from TP, GP, and glycidyl myristate after heating at 200 °C for 2 h.
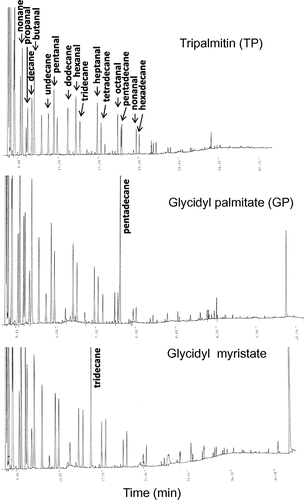
Fig. 5. Changes in the contents of saturated aldehydes from GP and TP during heating at 180 and 200 °C. ● GP (200 °C), ▴ GP (180 °C), ■ TP (200 °C), and ♦ TP (180 °C). Data show the mean and standard deviation of triplicate.
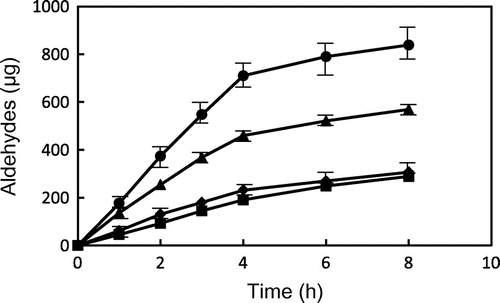
Fig. 6. Changes in the contents of hydrocarbons from GP and TP during heating at 180 and 200 °C. ● GP (200 °C), ▴ GP (180 °C), ■ TP (200 °C), and ♦ TP (180 °C). Data show the mean and standard deviation of triplicate.
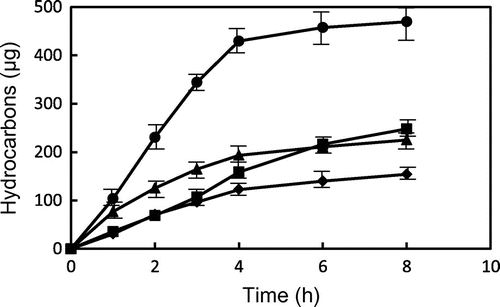
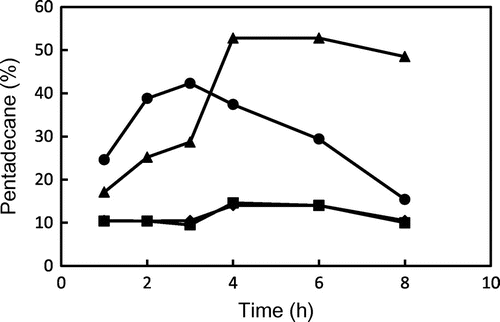
Fig. 7. Changes in the relative ratio of pentadecane to peak areas of total hydrocarbons from GP and TP during heating at 180 and 200 °C. ● GP (200 °C), ▴ GP (180 °C), ■ TP (200 °C), and ♦ TP (180 °C). Data show the mean and standard deviation of triplicate.