Figures & data
Fig. 1. SDS-PAGE analyses of crude and purified recombinant T3-3AP.
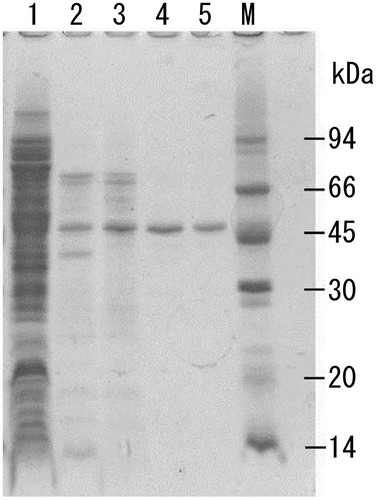
Table 1. Purification of recombinant T3-3AP.
Table 2. Comparison of activities toward luminescent substrates.
Fig. 2. Biophysical properties of recombinant T3-3AP Notes: (A) effect of pH on the stability of recombinant T3-3AP; enzymes were incubated at 25 °C for 24 h in acetate buffer (○), MES buffer (◆), triethanolamine buffer (□), and glycine buffer (▲). Residual activity was measured at pH 8.0 at 60 °C. Initial activity before incubation is defined as 100%. (B) Effect of temperature on the stability of recombinant T3-3AP (◆) and CIAP (□); APase solutions were incubated at various temperatures for 60 min and were then cooled, and residual activity was measured at pH 9.8 at 37 °C. Initial activities before incubation are defined as 100%. (C) Effect of pH on the activity of recombinant T3-3AP; measurements were performed in diethanolamine buffer at 37 °C. Activities under optimal pH conditions are defined as 100.
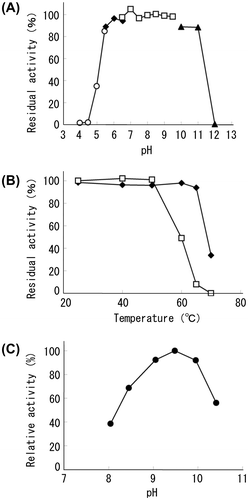
Fig. 3. Residual specific activity of wild type and mutant T3-3AP enzymes after maleimide activation.
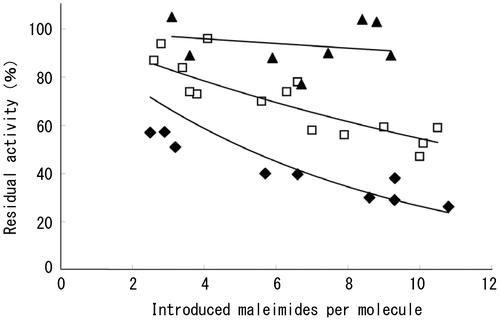
Fig. 4. Enzyme linked immunosorbent assays (ELISA) using APase-conjugated IgG.
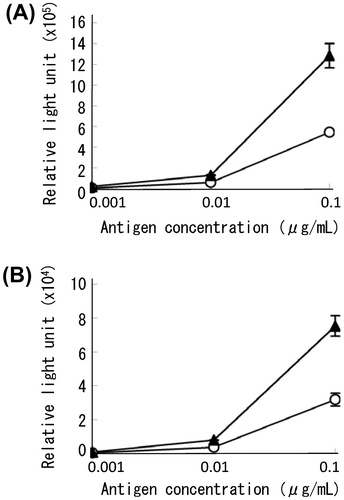
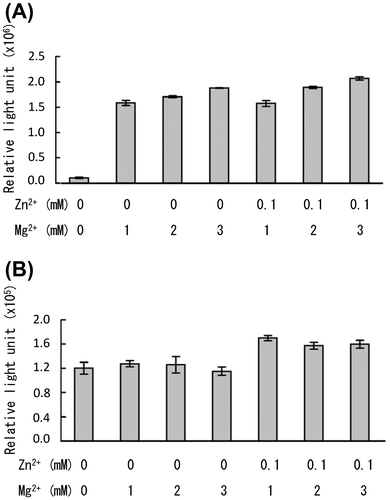
Fig. 5. Effects of Mg2+ and Zn2+ on ELISA reactivity.