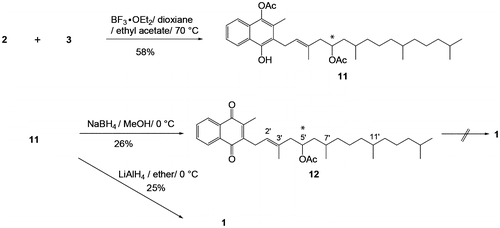
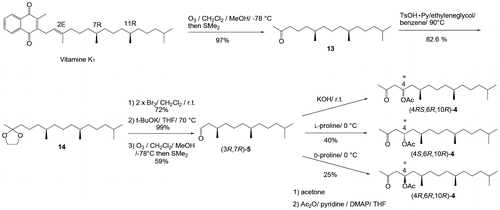
Figures & data

Fig. 1. 600 MHz 1H NMR spectrum of as-synthesized 5′-hydroxyphylloquinone (1) determined in CDCl3 using a JEOL ECA-600.
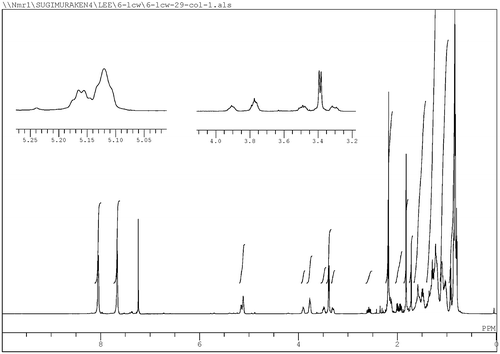
Fig. 2. Chiral GLC charts of stereocontrolled and uncontrolled products 4 derived from industrial VK1. (a) (4S)-enriched 4 derived from l-proline-catalyzed reaction, (b) (4R)-enriched 4 derived from d-proline-catalyzed reaction, (c) (4RS)-4 derived from VK1, and (d) stereoisomeric mixture of 4 prepared in Scheme .
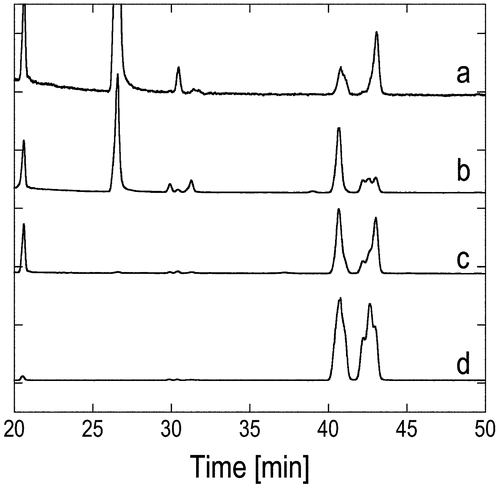
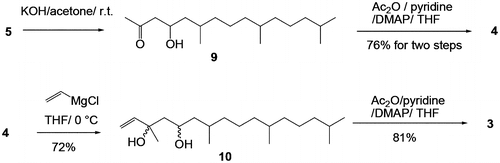
Fig. 3. TIC and mass chromatogram charts of compound 4 derived from natural 1 and stereo-controlled synthetic products 4 derived from industrial VK1. The TIC chart for (a) 4 derived from the natural product. Mass cromatograms at m/z 283 for 4 derived from the (b) natural product, (c) synthetic (4S)-4, (d) synthetic (4R)-4 and (e) synthetic (4RS)-4. The chart of (a) was integrated three times (n = 3) and smoothed using a seven-point weighted moving average.