Figures & data
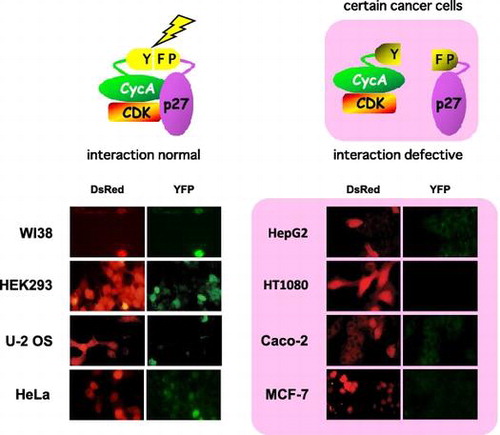
Fig. 1. Construction and verification of the split YFP system for detecting CycA-p27 interaction in living cells. (A) Construction of the split YFP vectors. PCMV, CMV promoter; MT, 6× myc epitope tag; L10, 10-amino acid linker peptide ([Gly-Gly-Gly-Gly-Ser]2). pCS2YC-L10-hp27 RXL carries five alanine-substitution mutations in the RXL (cyclin-binding) motif of p27. (B) Split-YFP fluorescence is detected specifically upon CycA-p27 interaction. HEK293 cells were transfected with 10 μg each of the indicated split-YFP (MT CycA-YFPN and YFPC-p27) expression vectors, and examined under fluorescent microscope. Nuclei were stained with DAPI. YFP signal was detected as green fluorescence. (C) MT CycA-YFPN and YFPC-p27 proteins form a functional complex. HEK293 cells were transfected with 10 μg each of the split-YFP expression vectors, and the cell extracts were subjected to anti-myc tag immunoprecipitation, followed by Western Blot Analysis using antibodies against the myc tag, p27 and CDK2, and by histone H1 kinase assay (32P-histone H1).
![Fig. 1. Construction and verification of the split YFP system for detecting CycA-p27 interaction in living cells. (A) Construction of the split YFP vectors. PCMV, CMV promoter; MT, 6× myc epitope tag; L10, 10-amino acid linker peptide ([Gly-Gly-Gly-Gly-Ser]2). pCS2YC-L10-hp27 RXL carries five alanine-substitution mutations in the RXL (cyclin-binding) motif of p27. (B) Split-YFP fluorescence is detected specifically upon CycA-p27 interaction. HEK293 cells were transfected with 10 μg each of the indicated split-YFP (MT CycA-YFPN and YFPC-p27) expression vectors, and examined under fluorescent microscope. Nuclei were stained with DAPI. YFP signal was detected as green fluorescence. (C) MT CycA-YFPN and YFPC-p27 proteins form a functional complex. HEK293 cells were transfected with 10 μg each of the split-YFP expression vectors, and the cell extracts were subjected to anti-myc tag immunoprecipitation, followed by Western Blot Analysis using antibodies against the myc tag, p27 and CDK2, and by histone H1 kinase assay (32P-histone H1).](/cms/asset/28f813bc-78b0-42a0-aa9d-c64477fe8595/tbbb_a_1391686_f0001_oc.gif)
Fig. 2. Evaluation of CycA-p27 interaction by split-YFP fluorescence in various cancer cell lines. WI-38, HEK293, U-2 OS, MDAH041, Kato Ⅲ, HeLa (A) and A431, Caco-2, HepG2, Saos-2, HT1080, MCF-7 (B) cells were co-transfected with 10 μg each of the split-YFP expression vectors and 1 μg of DsRed-expressing vector, and examined under fluorescent microscope. Nuclei were stained with DAPI. Note that the cells in (B) showed no detectable YFP signal.
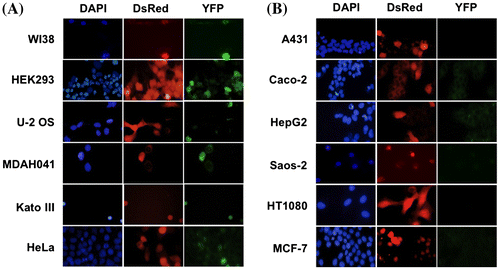
Fig. 3. Absence of YFP signal in HT1080 and HepG2 cells is independent of the expression level of split-YFP fusion proteins. (A) HEK293, U-2 OS, HT1080, MDAH041, HeLa, HepG2 cells were co-transfected with 10 μg each of the split-YFP and myc-tagged (MT) LacZ expression vectors. The cell extracts were immunoblotted using antibodies against myc tag and p27. (B) HT1080 and HepG2 cells were co-transfected with 10 μg each of the split-YFP and MT LacZ expression vectors in the absence and presence of 10 μM proteasome inhibitor MG132 and immunoblotted using antibodies against myc tag. (C) HEK293, HT1080 and HepG2 cells were co-transfected with 10 μg each of the split-YFP and 1 μg of DsRed-expressing vectors in the absence and presence of 10 μM MG132, and examined under fluorescent microscope. Nuclei were stained with DAPI.
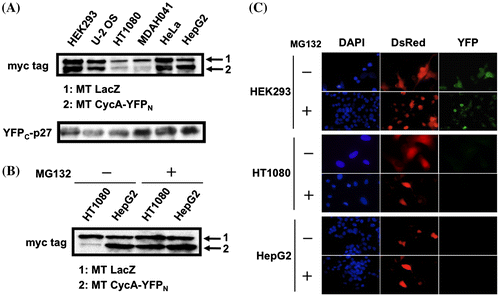
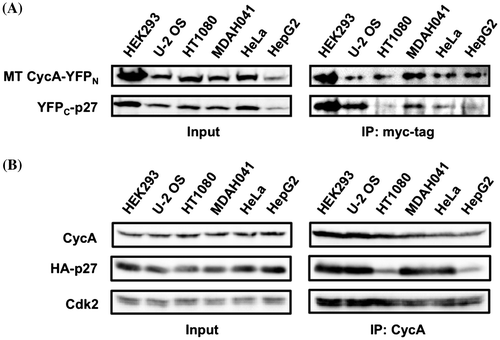
Fig. 4. Co-immunoprecipitation of CycA and p27 proteins in various cancer cell lines. (A) HEK293, U-2 OS, HT1080, MDAH041, HeLa, and HepG2 cells were co-transfected with 10 μg each of the split-YFP expression vectors (10 μM MG132 was added for HT1080 and MDAH041), and the cell extracts were subjected to anti-myc tag immunoprecipitation, followed by Western Analysis using antibodies against myc tag and p27. (B) The above six cell lines were transfected with 1 μg (HEK293), 2 μg (HT1080), 3 μg (U-2 OS, HeLa) or 5 μg (MDAH041, HepG2) of HA-p27 expression vector to equalize the expression level, and the cell extracts were subjected to anti-CycA immunoprecipitation, followed by Western Analysis using antibodies against CycA, HA tag, and Cdk2. See Supplementary Fig. S4 for quantified data.