Figures & data
Table 1. Bacterial strains used in this study.
Figure 2. Restriction maps of the plasmids used in this study. Boxes denote the vector regions. The small arrowheads and the gray areas in the boxes denote the promoters and vector-derived coding regions for hexahistidine tags. Large solid arrows denote the coding regions of the ispA, ispB, and ispU/rth genes. Small arrows denote primer binding sites for PCR. Abbreviations: E, EcoRI site; H, HindIII site; S, SalI site; V, EcoRV site; Plac, lac promoter; Ptrc, trc promoter.
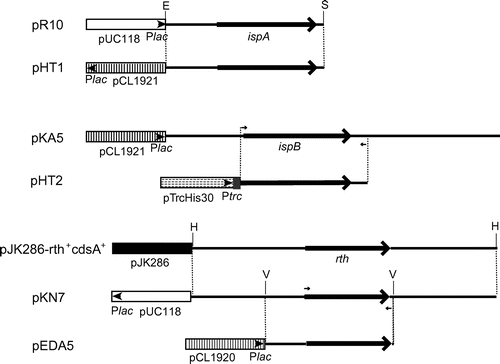
Table 2. Growth rates of strains and transformants used in this study.
Figure 3. The level of farnesyl diphosphate (FPP) in strains and transformants used in this study. SF5 (the wild-type strain), SF7N (the ispA-null mutant), SF7N harboring pHT1 (which carries ispA), and SF7N harboring pKA5 (which carries ispB) were used for the quantification of FPP. Bar graphs show averages of three assays. Error bars show standard deviations. The statistical significance is indicated (*p < 0.05 compared with SF7N; **p < 0.01 compared with SF7N).
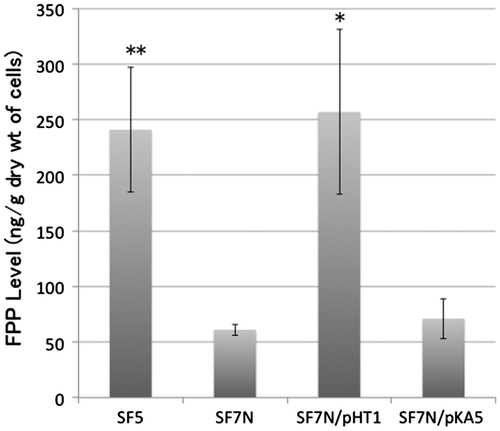
Figure 4. High-performance liquid chromatography-mass spectrometry analysis of the ubiquinone fraction of SF7N. (A) Chromatogram of absorbance detected at 275 nm. Peak a, ubiquinone-8; peak b, ubiquinone-9; peak c, ubiquinone-10. (B) Mass spectrum of peak b.
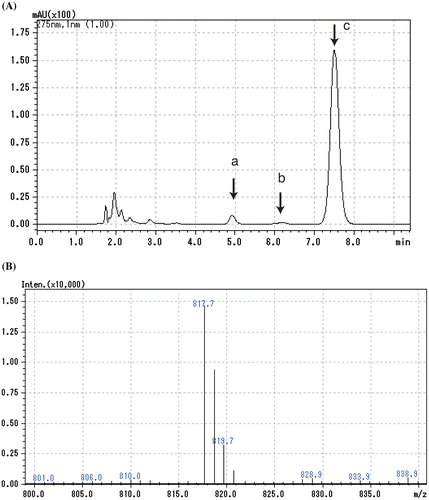
Figure 5. The level of isoprenoid quinones and undecaprenyl phosphate in transformants of ispA null mutant E. coli strain SF7N. The transformants of SF7N, harboring pCL1921 (empty vector), pHT1 (which carries ispA), pKA5 (which carries ispB), and pEDA5 (which carries ispU/rth) were used for the analysis of isoprenoids. Bar graphs show averages of three assays. Error bars show standard deviations. The statistical significance is indicated (**p < 0.01 compared with SF7N/pCL1921). Abbreviations: UQ-8, ubiquinone-8; UQ-9, ubiquinone-9; MK-8, menaquinone-8; UP, undecaprenyl phosphate.
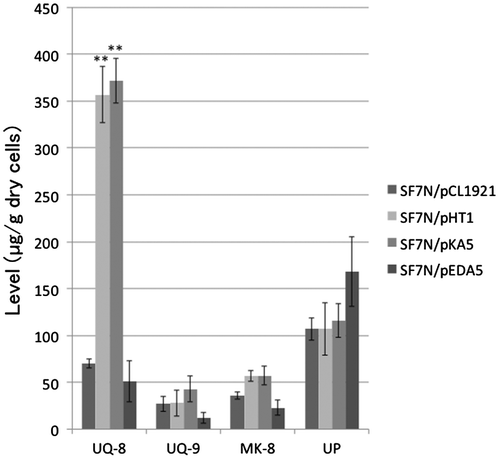
Table 3. Activity of hexahistidine-tagged octaprenyl diphosphate synthase.
Figure 6. Autoradiograms of thin layer chromatography (TLC) of prenyl alcohols obtained by enzymatic hydrolysis. Reaction of [1-14C]IPP and DMAPP was catalyzed by purified hexahistidine-tagged IspB. The products were extracted with butanol and treated with acid phosphatase. The resulting polyprenols were extracted with hexane and analyzed by normal phase TLC on a silica gel 60 plate using benzene-ethyl acetate (4:1 v/v) (A) and by reversed phase TLC on an LKC-18 plate using acetone-water (9:1 v/v) (B). Abbreviations: Ori, Origin; SF, solvent front; C15, E,E-farnesol; C20, E,E,E-geranylgeraniol; C45, solanesol (all-E-nonaprenol); C50, ficaprenol-50 (Z,E-mixed decaprenol); C55, ficaprenol-55 (Z,E-mixed undecaprenol); C60, ficaprenol-60 (Z,E-mixed dodecaprenol).
![Figure 6. Autoradiograms of thin layer chromatography (TLC) of prenyl alcohols obtained by enzymatic hydrolysis. Reaction of [1-14C]IPP and DMAPP was catalyzed by purified hexahistidine-tagged IspB. The products were extracted with butanol and treated with acid phosphatase. The resulting polyprenols were extracted with hexane and analyzed by normal phase TLC on a silica gel 60 plate using benzene-ethyl acetate (4:1 v/v) (A) and by reversed phase TLC on an LKC-18 plate using acetone-water (9:1 v/v) (B). Abbreviations: Ori, Origin; SF, solvent front; C15, E,E-farnesol; C20, E,E,E-geranylgeraniol; C45, solanesol (all-E-nonaprenol); C50, ficaprenol-50 (Z,E-mixed decaprenol); C55, ficaprenol-55 (Z,E-mixed undecaprenol); C60, ficaprenol-60 (Z,E-mixed dodecaprenol).](/cms/asset/7a443bfb-8007-4d70-8d8a-e4eb7d78dacb/tbbb_a_1398066_f0006_oc.gif)