Figures & data
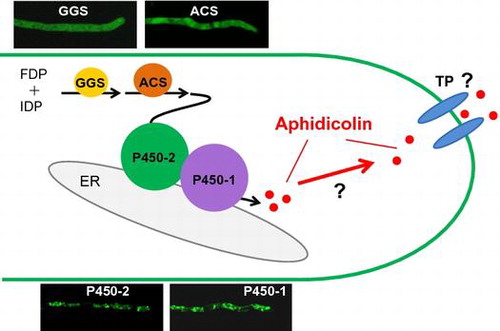
Figure 2. Subcellular localization of PbGGS and PbACS in A. oryzae. Hyphae of strains expressing GFP-PbGGS (A) and PbACS-GFP (B) were grown for 20 h in minimal medium (MM) containing 1% maltose as the sole carbon source. GFP fluorescence at subapical and apical regions was observed by confocal microscopy.
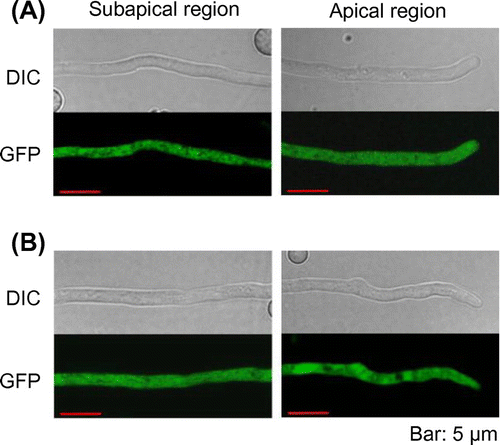
Figure 3. Subcellular localization of PbP450-1 and PbP450-2 in A. oryzae. Hyphae of strains expressing PbP450-1-GFP (A and C) and PbP450-2-GFP (B, D, and E) with mCherry-fused organelle markers were examined by confocal microscopy. (F) Colocalization analysis of GFP-fused PbP450-1 and mCherry-fused PbP450-2. Fluorescence images of apical and subapical regions are shown in the right and left panels, respectively.
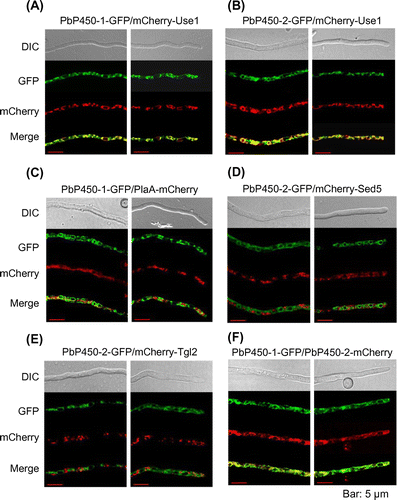
Figure 4. Protease protection assay of GFP-fused Pb450s. Mycelia grown for 36 h in MM containing 1% casamino acids as the sole carbon source were transferred to fresh MM containing 1% maltose and incubated for 6 h. The homogenates were prepared from harvested mycelia and treated with proteinase K with or without Triton X-100. Protected proteins from proteinase K digestion were detected by western blot analysis using an anti-GFP antibody.
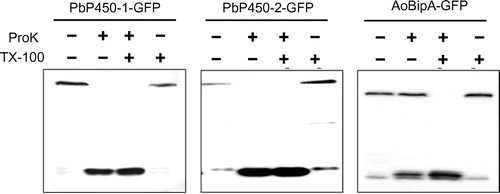
Figure 5. Subcellular localization of PbP450-1 and PbP450-2 mutants lacking the transmembrane domain. Hyphae of PbP450-2∆TM-GFP (A) and PbP450-1∆TM-GFP (B) expressing strains were examined by confocal microscopy. (C) Protease protection assay of Pb450-1∆TM-GFP.
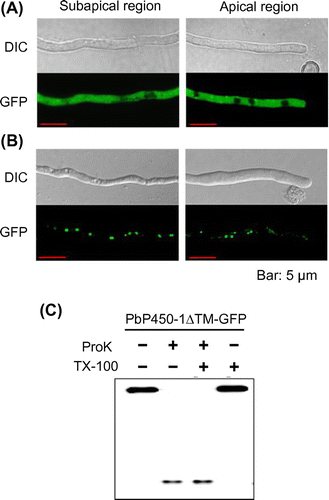
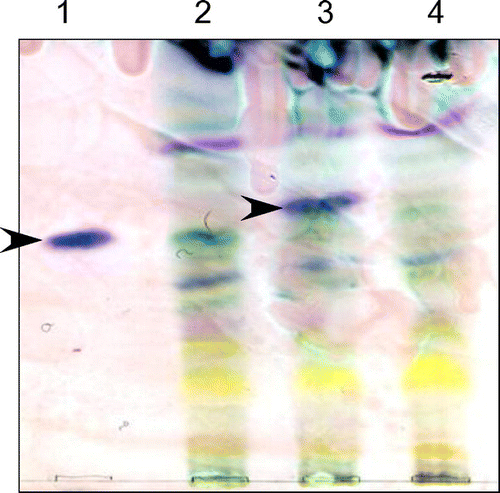
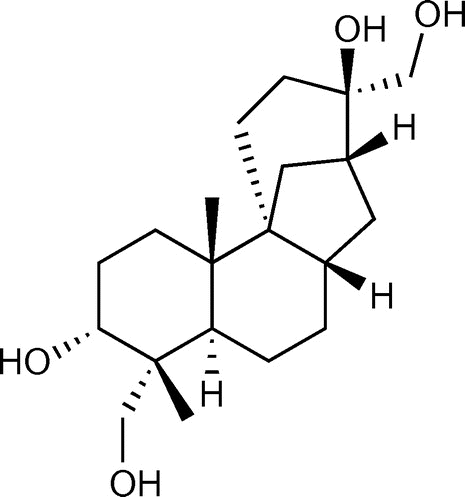
Figure 6. Detection of aphidicolin production by thin-layer chromatography. A. oryzae strains were cultured in MM with 1% tryptone and 3% maltose for 6 days. Aphidicolin was extracted from the culture supernatant and mycelium with ethyl acetate. The reagent product of aphidicolin (lane 1) was used as a control. An A. oryzae strain expressing PbGGS, PbACS, Pb450-2, and PbTP (lane 2) was used as a host for the expression of intact PbP450-1 (lane 3) and PbP450-1∆TM (lane 4). Arrowheads indicate the signal corresponding to aphidicolin.