Figures & data
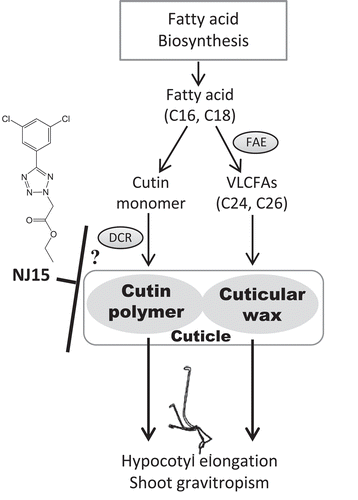
Figure 1. f127 showed lower sensitivity to NJ15 than WT Arabidopsis.
Seeds of WT and f127 plants were grown vertically in 1/2 MS medium containing NJ15 at the concentrations indicated in the figure. After four days, these plates were turned 90° from their initial orientation and allowed to grow for one day. Then, (a) hypocotyl length and (b) gravitropic response of the shoots were measured. Data are means ± SD (n = 12). Experiments were repeated three times independently, and similar results were obtained.
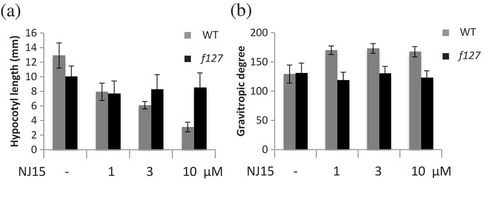
Figure 2. Low-sensitivity of f127 to NJ15 is caused by loss-of-function of DCR.
(a) With TAIL-PCR analysis of f127 mutants, insertion of T-DNA was detected in the second exon of the DCR gene. (b) Expression analysis of DCR (left) and At4g11100 (right). (c) & (d) Cuticle permeability stained with toluidine blue-O (TB). Etiolated seedlings (c) grown in the negative control, and (d) in the medium containing NJ15 (10 µM) were used. Staining was repeated three times with similar results. Scale bars are 5 mm.
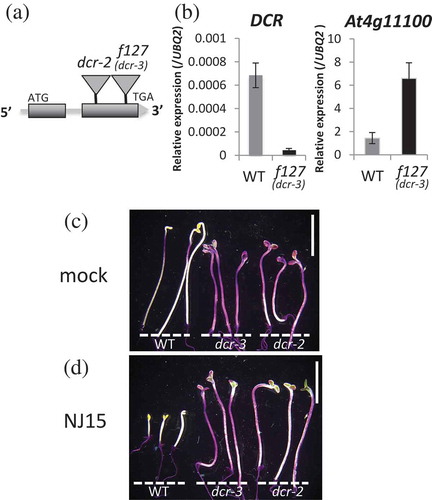
Figure 3. The numbers of NJ15-responsive genes are decreased in dcr-3.
(a) The Venn diagram shows the overlap between the sets of differently expressed genes (DEGs) in NJ15-treated WT (WT-NJ15) vs. those in WT-mock [WT-NJ15/WT-mock], [(dcr-3)-NJ15/(dcr-3)-mock], and [(dcr-3)-mock/WT-mock] plants. Four-day-old seedlings grown in the dark were treated with 10 μM of NJ15 or mock substance (DMSO). Then, they were subjected to gravitropic stimulus for 3 h before RNA extraction. (b) Comparison of Gene Ontology (GO) profiles of upregulated and downregulated DEGs in a set of common 139 genes between WT-NJ15/WT-mock and (dcr-3)-NJ15/(dcr-3)-mock plants. Terms were adopted at the second level of biological process ontology. Y-axis bar is the frequency of each enriched GO term.
![Figure 3. The numbers of NJ15-responsive genes are decreased in dcr-3.(a) The Venn diagram shows the overlap between the sets of differently expressed genes (DEGs) in NJ15-treated WT (WT-NJ15) vs. those in WT-mock [WT-NJ15/WT-mock], [(dcr-3)-NJ15/(dcr-3)-mock], and [(dcr-3)-mock/WT-mock] plants. Four-day-old seedlings grown in the dark were treated with 10 μM of NJ15 or mock substance (DMSO). Then, they were subjected to gravitropic stimulus for 3 h before RNA extraction. (b) Comparison of Gene Ontology (GO) profiles of upregulated and downregulated DEGs in a set of common 139 genes between WT-NJ15/WT-mock and (dcr-3)-NJ15/(dcr-3)-mock plants. Terms were adopted at the second level of biological process ontology. Y-axis bar is the frequency of each enriched GO term.](/cms/asset/65ef8fd1-c978-4837-ad9c-8ae9d92f7ba2/tbbb_a_1484278_f0003_b.gif)
Figure 4. Effects of NJ15 and Cafenstrole on hypocotyl length and gravitropic response of seedlings grown in the dark.
Seedlings were grown vertically on 1/2 MS medium containing the indicated chemicals. After 4 days, plates were turned 90° from the initial orientation and then continued growing for one day. Then, (a) the hypocotyl length and (b) gravitropic response of seedlings were measured. Data are means ± SD (n = 20). Experiments were repeated three times independently, and similar results were obtained. Asterisks indicate significant differences (*, 0.01 < p < 0.05; **, p < 0.01; Student’s t-test).
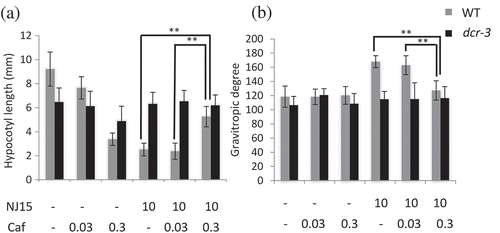
Figure 5. Effects of NJ15 and cafenstrole on an endogenous fatty acid fluctuation in etiolated seedlings.
Five-day-old seedlings grown on 1/2 MS medium containing the indicated chemicals were subjected to the quantification of several kinds of fatty acids with GC/MS. Relative contents of (a) Palmitic acid (C16:0), (b) palmitoleic acid (C16:1), (c) stearic acid (C18:0), (d) oleic acid (C18:1), (e) linoleic acid (C18:2), (f) alpha-linolenic acid (C18:3), (g) behenic acid (C22:0), (h) lignoceric acid (C24:0), and (i) cerotic acid (C26:0) were quantified with exogenously applied margaric acid (C17:0) as an internal standard. Data are means ± SD (n = 3). Asterisks indicate significant differences (*, 0.01 < p < 0.05; **, p < 0.01; Student’s t-test).
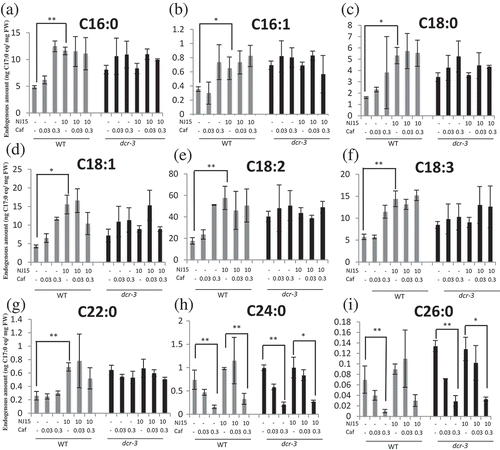
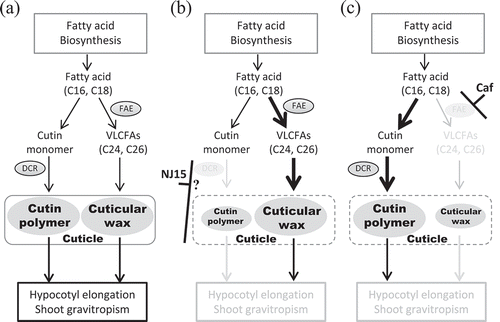
Figure 6. A possible mechanism for low sensitivity to NJ15 in dcr-3.
(a) In WT Arabidopsis, fatty acid (FA) biosynthesis contributes to hypocotyl elongation and shoot gravitropism via at least two routes. One is the formation of cutin polymer from monomers in the cuticle (left route), catalyzed by DCR. The other (right route) is the formation of cuticular wax in the cuticle from very-long-chain FAs (VLCFAs). (b) The dcr-3 mutant lacks functional DCR, which affects the formation of cutin polymer. As a result, the contribution of the left route for hypocotyl elongation and shoot gravitropism should decrease in dcr-3, which leads to low sensitivity to NJ15. We proposed that the target of NJ15 activity is likely around the formation of cutin polymer because NJ15 had no effect on the amount of VLCFAs as well as those of C16 and C18 fatty acids in dcr-3. (c) Cafenstrole targets the right route, which affects the formation of cuticular wax.