Figures & data
Figure 1. The contribution of the C-terminus of Arabidopsis Cat2 to its subcellular localization.
Arabidopsis protoplasts were co-transfected with plant expression vectors under control of the constitutive CaMV35S promoter with the sequence coding Arabidopsis AtCat2 fused with GFP and peroxisomal marker; RFP-PTS1 or RFP-AtPEX5. The signals of GFP (left) and RFP (middle) were observed, and they were merged (right). (a) Upper three-row panels; GFP only (GFP) or GFP fused at the N-terminus (GFP-AtCat2) and C-terminus (AtCat2-GFP) of AtCat2 were transiently expressed with RFP-PTS1. Lower two-row panels; GFP-PTS1 or GFP-AtCat2 was transiently expressed with RFP-AtPEX5. (b) Upper part shows the representation of the C-terminal amino acids of AtCat2 and its mutants. Lower part shows the signals of GFP and RFP in the transfected protoplasts. The amino acids (QKLA) of GFP-AtCat2 at the positions 480 to 483 from the N-terminus of AtCat2 were substituted to Gly (Q480G, K481G, L482G and A483G, respectively). (c) Upper part shows the representation of the C-terminal amino acids of AtCat2 and its truncated mutants. Lower part shows the signals of GFP and RFP in the transfected protoplasts. The C-terminal 3, 9, 10 and 11 amino acids of AtCat2 were deleted from GFP-AtCat2 (ΔC3, ΔC9, ΔC10 and ΔC11, respectively). Scale bars = 5 µm.
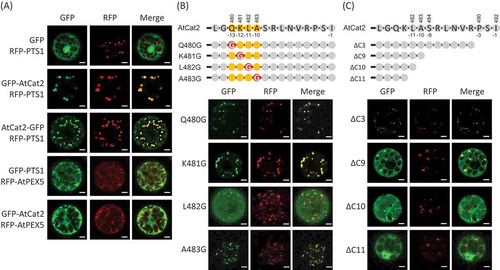
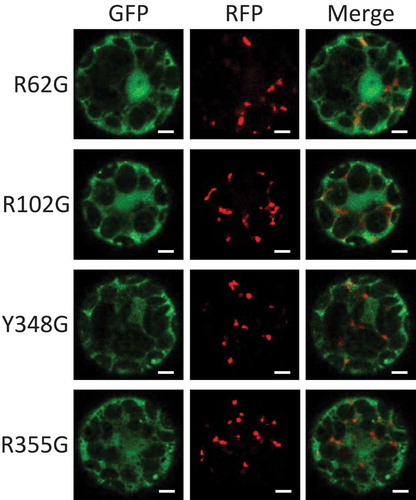
Figure 2. The role of the amino acids related to heme binding of Arabidopsis Cat2 in its subcellular localization.
Arabidopsis AtCat2 with GFP fused at the N-terminus and RFP-PTS1 were transiently expressed under control of constitutive CaMV35S promoter in Arabidopsis protoplasts. The transfected protoplasts were observed for signals of GFP (left) and RFP (middle), and they were merged (right). The four amino acids (positions 62, 102, 348 and 355 from the N-terminus of AtCat2) related to heme binding were substituted with Gly (R62G, R102G, Y348G and R355G, respectively). Scale bars = 5 µm.