Figures & data
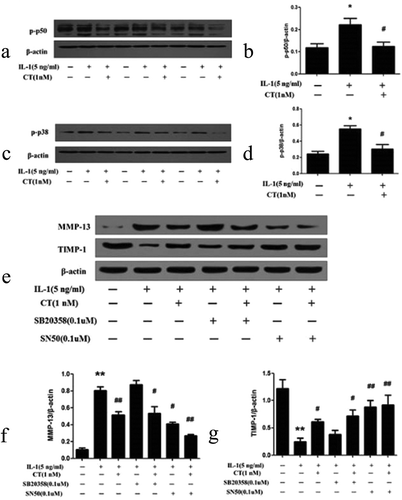
Figure 1. Compared to non IL-1β stimulated human chondrocytes, CT has no significant effects on cell viability at concentrations (0.1, 0.5, 1, 5, 10 and 50 nM) after 24 h treatment in IL-1β stimulated human chondrocytes.
The values were presented as mean ± SD and there were no significant differences between each groups (P > 0.05). CT: calcitonin; IL-11β: interleukin-11β.
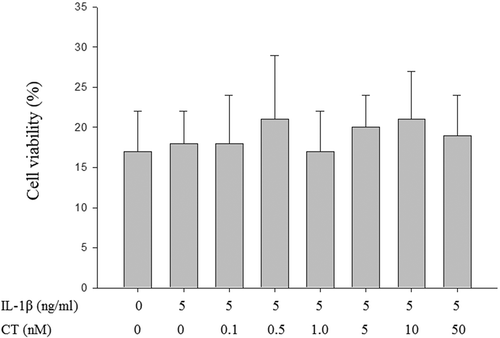
Figure 2. CT inhibits expression of MMP-13 in IL-1β stimulated human chondrocytes.
(a) Western blotting analysis of MMP-13; (b) Relative quantitation of MMP-13 expression; ** P < 0.01 compared with the control group (** vs. *); # P < 0.05 compared with the IL-1β group (# vs. **); ##P < 0.01 compared with the IL-1β group (## vs.**); CT: calcitonin; IL-1β: interleukin-1β; MMP-13: matrix metalloproteinases-13.
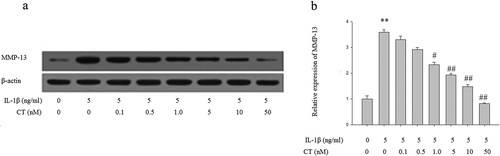
Figure 3. CT increases expression of TIMP-1 in IL-1β stimulated human chondrocytes. (a) Western blotting analysis of TIMP-1; (b) Relative quantitation of TIMP-1 expression; ** P < 0.01 compared with the control group (** vs. *); # P < 0.05 compared with the IL-1β group (# vs. **); CT: calcitonin; IL-1β: interleukin-1β; TIMP-1: tissue inhibitors of metalloproteinases-1.
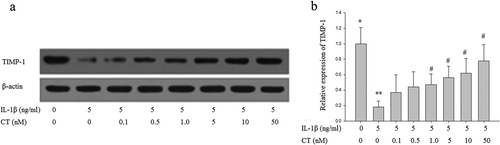
Figure 4. Effect of CT on the ratio of MMP-13/TIMP-1 in IL-1β stimulated human chondrocytes.
The ratio of MMP-13/TIMP-1 in the IL-1β stimulated group was significantly higher than in the control group (** vs. *, P < 0.05). The ratio of MMP-13/TIMP-1 at the CT doses of 1, 5, 10 and 50 nM were significantly decreased compared to non CT pretreated cells (## vs. **, , P < 0.05). CT: calcitonin; IL-1β: interleukin-1β; MMP-13: matrix metalloproteinases-13; TIMP-1: tissue inhibitors of metalloproteinases-1.
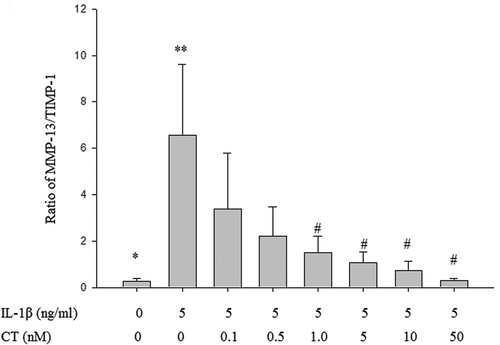
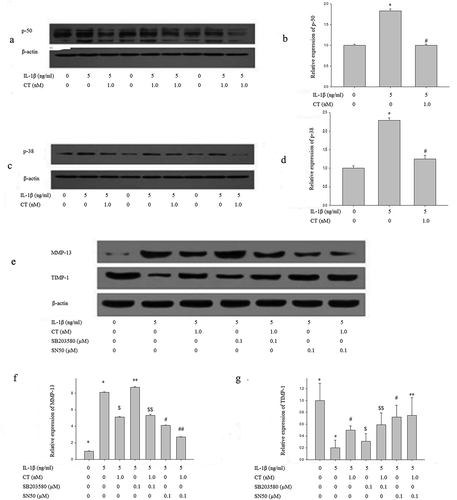
Figure 5. CT regulates expression of MMP 13/TIMP-1 via p50-NF-κB pathway.
(a-b) Western blotting analysis and relative quantitation of p50 expression in IL-1β stimulated human chondrocytes; (c-d) Western blotting analysis and relative quantitation of p38 expression in IL-1β stimulated human chondrocytes; (e-g) Western blotting analysis and relative quantitation of MMP-13 and TIMP-1 expression in IL-1β stimulated human chondrocytes pretreated with SN50 (0.1 μM) or SB203580 (0.1 μM). (e) and (f) showed IL-1β significantly stimulated MMP-13 expression (*) compared to the control group (^) (P < 0.01), CT inhibited IL-1β stimulated high expression of MMP-13 ($) compared to the IL-1β stimulated cells (*) (P < 0.01). After adding SB203580, the MMP-13 expression unchanged (IL-1β-SB203580 treated cells ** vs. IL-1β treated cells * and IL-1β-CT-SB203580 treated cells $$ vs. IL-1β-CT treated cells $, P > 0.05). After adding SN50, IL-1β stimulated the high expression of MMP-13 was significantly decreased (IL-1β-SN50 treated cells # vs. IL-1β treated cells *, P < 0.05), and the expression level of MMP-13 was further significantly decreased (IL-1β-CT-SN50 treated cells ## vs. IL-1β-CT treated cells $, P < 0.05). (e) and (g) showed IL-1β significantly inhibited the expression of TIMP-1 (IL-1β treated cells * vs. the control cells ^, P < 0.01), CT enhanced the expression of TIMP-1 inhibited by IL-1β (IL-1β-CT treated cells # vs. IL-1β treated cells *, P < 0.05). SB203580 increased the expression of TIMP-1 inhibited by IL-1β, but no statistical difference (IL-1β-SB203580 treated cells $ vs. IL-1β treated cells * and IL-1β-CT-SB203580 treated cells $$ vs. IL-1β-CT-SN50 treated cells #, P > 0.05). The expression of TIMP-1 inhibited by IL-1β increased significantly after use of SN50 (IL-1β-SN50 treated cells # vs. IL-1β-treated cells * and IL-1β-CT-SN50 treated cells ** vs. IL-1β-SN50 treated cells #, P < 0.01). CT regulates expression of MMP 13/TIMP-1 via p50-NF-κB pathway.