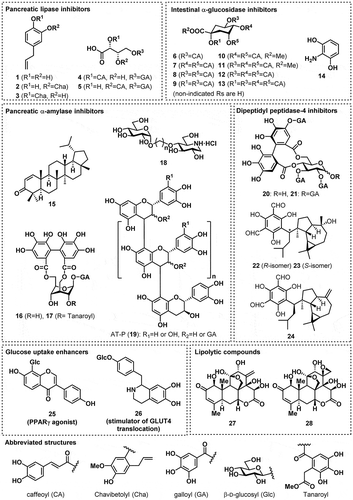
Figures & data
Figure 2. α-Amylase inhibitory activity of artificial inhibitors (18). The numbers indicate the length of the alkyl linker between deoxynojirimycin and the glucose moiety indicated by “n” in structure of compound 18 ().
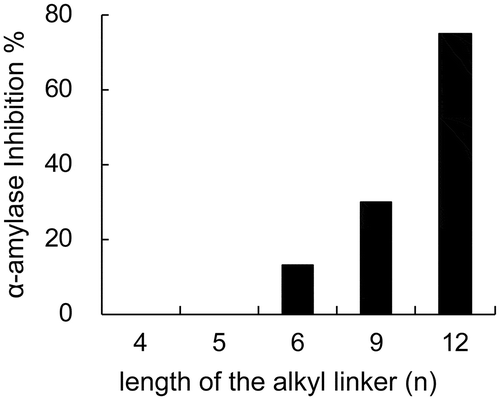
Table 1. α-Amylase inhibitory activity of proanthocyanidins isolated from plants.
Figure 3. The mechanism of the higher glucose uptake induced by higenamine 4ʹ-O-β-D-glucoside (26). Compound 26 activates the β2AR to increase glucose uptake. The proteins in boxes were evaluated for their involvement and the other major pathways colored gray were not implicated. β2AR: β2-adrenergic receptor, AC: adenylyl cyclase, cAMP: cyclic adenosine monophosphate, PKA: cAMP-activated protein kinase A, mTORC2: mechanistic target of rapamycin complex-2, GLUT4: glucose transporter 4, IR: insulin receptor, IRS: insulin receptor substrate, PI3K: phosphoinositide 3-kinase, PKB (AKT): protein kinase B, AMPK: AMP-activated protein kinase.
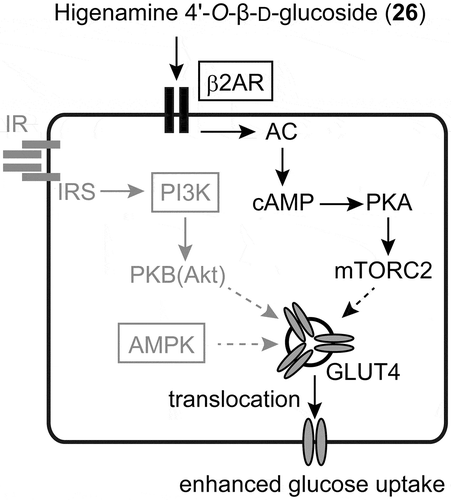
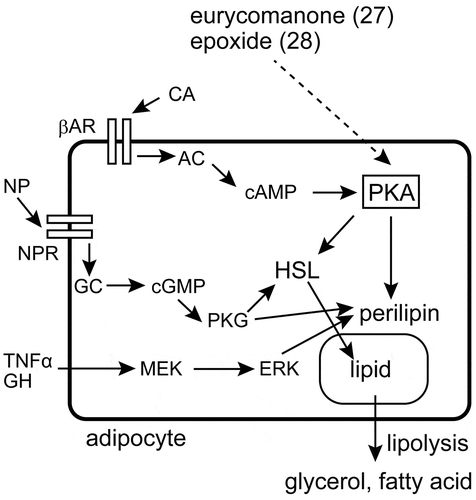
Figure 4. Lipolytic pathways and the target of eurycomanone (27) and its epoxide (28). Compounds 27 and 28 have their effect through the activation of PKA. The involvement of ERK in this activity has been ruled out, but other proteins involved in lipolysis have not been studied in detail. AC: adenylyl cyclase, βAR: β-adrenergic receptor, CA: catecholamine, cAMP: cyclic adenosine monophosphate, cGMP: cyclic guanosine monophosphate, ERK: extracellular signal-regulated kinase, GC: guanylyl cyclase, GH: growth hormone, HSL: hormone-sensitive lipase, MEK: MAPK/ERK kinase, NP: natriuretic peptide, NPR: natriuretic peptide receptor, PKA: cAMP-activated protein kinase, PKG: cGMP-activated protein kinase, TNFα: tumor necrosis factor-α.