Figures & data
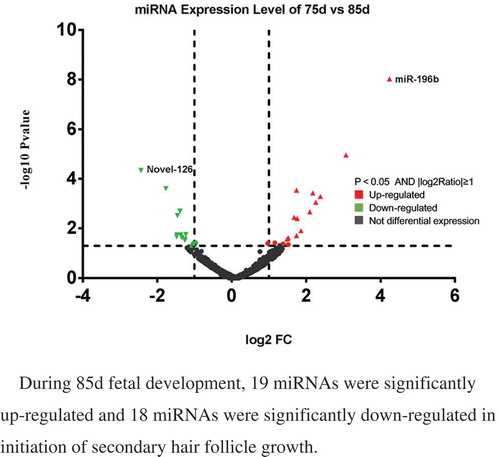
Table 1. The primers of real-time PCR.
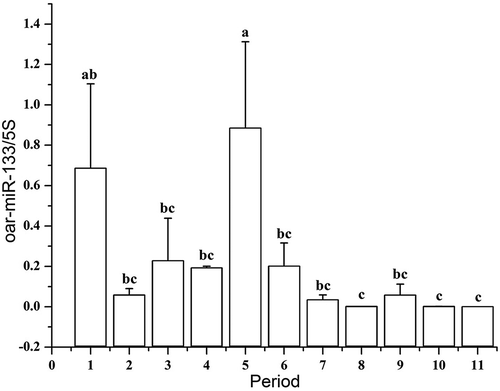
Figure 1. Variation in process of secondary hair follicle morphogenesis in Chinese Merino sheep during the foetal period.
A: 75 days of foetal development (100×); B: 85 days of foetal development (100×) a1: Hair germ cells, a2: Dermal fibroblasts, PF: Primary hair follicle, SF: Secondary hair follicle
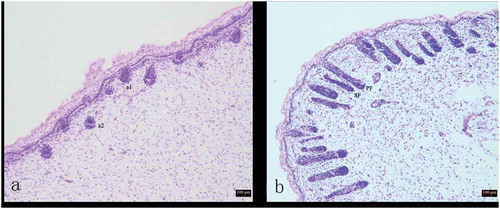
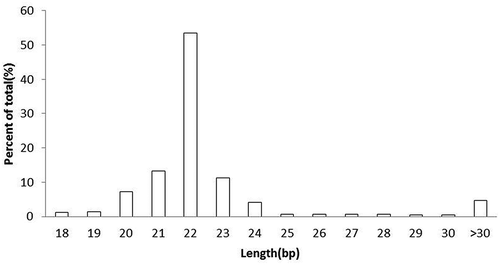
Table 2. Small RNA sequence statistics for 11 libraries.
Table 3. Expression Profile of Sequenced Reads in 11 libraries.
Table 4. The 10 most abundantly expressed known miRNAs in the skin and hair follicles.
Table 5. The 10 most abundantly expressed conversed miRNAs in the skin and hair follicles.
Table 6. The 10 most abundantly expressed novel miRNA in the skin and hair follicles.
Table 7. The 10 most abundantly expressed known miRNA in 75d and 85d of sheep hair follicles development.
Table 8. The 10 most abundantly expressed conversed miRNA in 75d and 85d of sheep hair follicles development.
Table 9. The 10 most abundantly expressed novel miRNA in 75d and 85d of sheep hair follicles development.
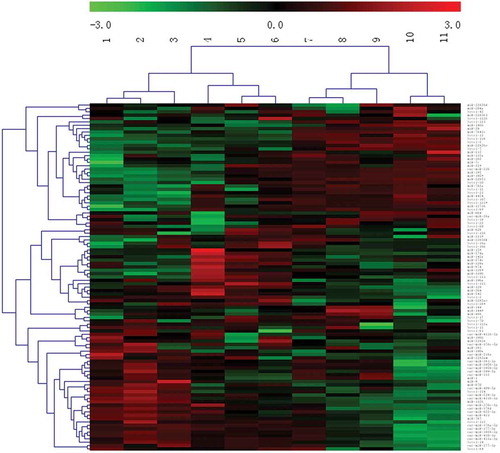
Figure 3. Differentially expressed miRNAs in sheep skin between 75 and 85 d of secondary HF morphogenesis.
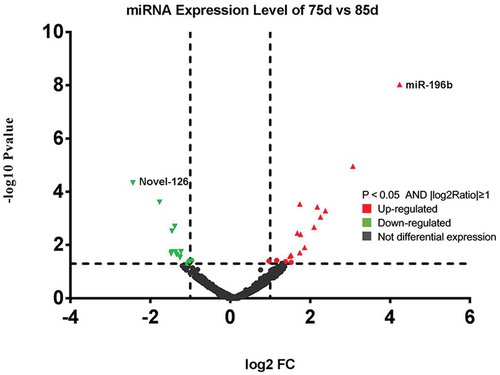
Table 10. Gene ontology analysis of differentially expressed genes in 11 periods development of sheep skin HF morphogenesis.
Table 11. Gene ontology analysis of differentially expressed genes in sheep skin between 75d and 85d of secondary HF morphogenesis.
Table 12. KEGG Pathway analysis of target genes of differentially expressed miRNA in 11 periods development of sheep skin HF morphogenesis.
Table 13. KEGG Pathway analysis of target genes of differentially expressed miRNA in sheep skin between 75d and 85d of secondary HF morphogenesis.
Table 14. Predicted target genes of prolactin signaling pathway and platelet activation in sheep skin between 75d and 85d of secondary HF morphogenesis.
Figure 5. Differentially expressed genes between 75 d and 85 d of secondary HF morphogenesis involved in prolactin signalling pathway.
Note: The red-labelled genes were significantly upregulated at 75 d compared with expression at 85 d (P-value<0.05). The blue-labelled genes were significantly downregulated at 75 d compared with expression at 85 d (P-value<0.05). The green-labelled genes were expressed in sheep skin during secondary HF initiation, but there were no significant differences.
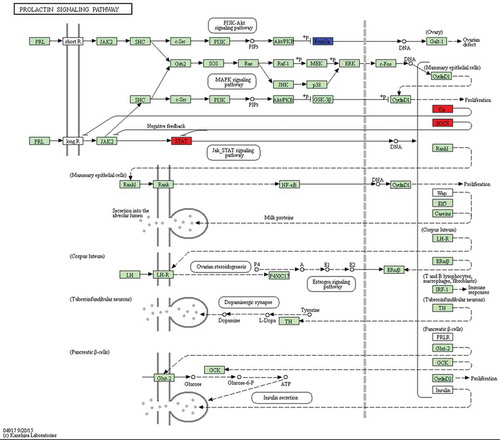
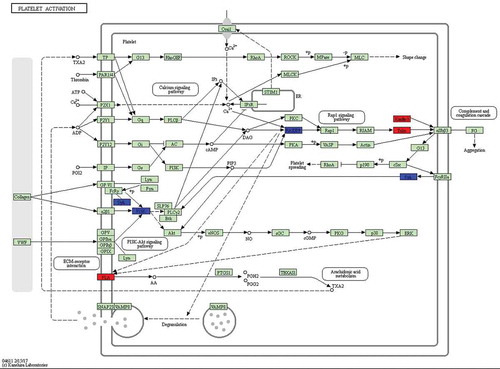
Figure 6. Differentially expressed genes between 75 d and 85 d of secondary HF morphogenesis involved in platelet activation.
Note: The red-labelled genes were significantly upregulated at 75 d compared with expression at 85 d (P-value<0.05). The blue-labelled genes were significantly downregulated at 75 d compared with expression at 85 d (P-value<0.05). The green-labelled genes were expressed in sheep skin during secondary HF initiation, but there were no significant differences.