Figures & data
Figure 1. Biosynthesis of nonproteinogenic amino acids used as building blocks of nonribosomal peptides. Biosynthesis pathways of methylproline (2) (a), α-methyl-l-serine (8) (b) and nitrotyrosine (10) (c) used as building blocks of griselimycin (1), JBIR-34 (6), −35 (7) and rufomycin (9), respectively, are shown. KG, alpha-ketoglutarate; 5,10-MTHF, 5,10-methylenetetrahydrofolate; THF, tetrahydrofolate; SC, succinate.
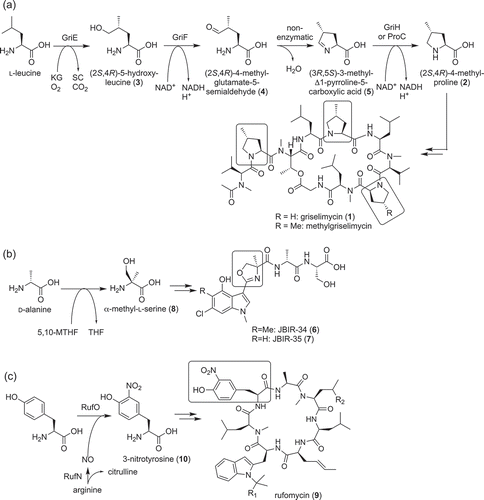
Figure 2. Biosynthesis of polyketides, streptazone E (11) (a), JBIR-76 (12), and −77 (13) (b) and fogacins (15–17) (c).
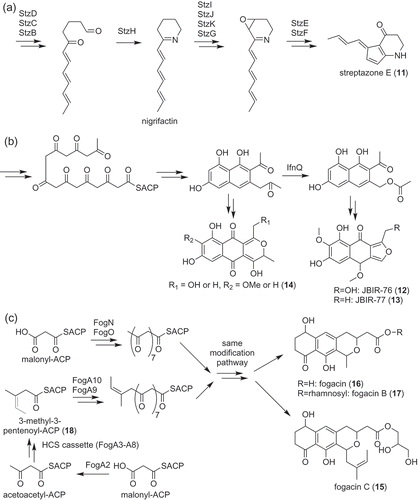
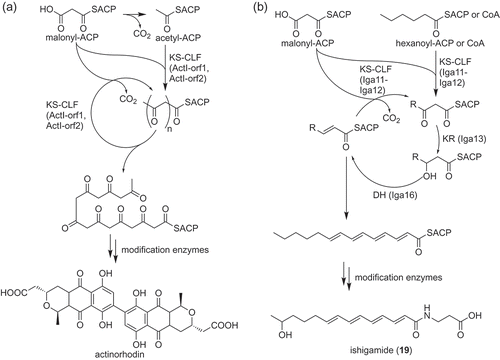
Figure 3. Biosynthesis of an aromatic polyketide and a polyene polyketide by type II PKS systems. Overview of biosynthetic pathways of actinorhodin (a) and ishigamide (19) (b) are shown as examples, respectively.