Figures & data
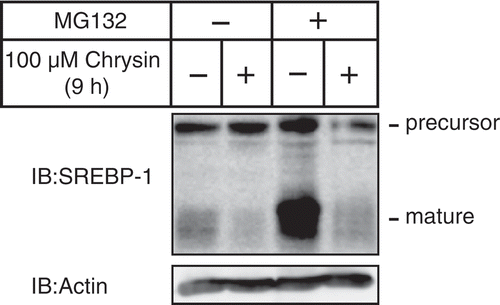
Figure 1. Chrysin reduces the activity of the SRE-containing FAS promoter.
(a) Chrysin structure (b) Huh-7/Fas-luc cells were depleted of sterols through a 16 h incubation in medium B. The cells were then switched to medium B in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After a 16 h incubation, a luciferase assay was performed and the relative activity of luciferase was obtained by normalization to the activity in the presence of vehicle. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).
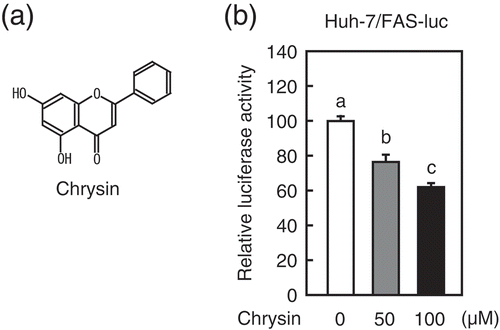
Figure 2. Chrysin reduces SREBP target gene expression and de novo synthesis of fatty acids and cholesterol.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a) The cells were then switched to medium C in the presence of either vehicle or the indicated chrysin concentrations. After a 24 h incubation, total RNA was isolated from the cells. Quantitative real-time PCR was performed, and the relative mRNA levels were obtained by normalization to GAPDH. (b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After an 18 h incubation, the cells were treated with 1.6 μCi/mL [Citation14C]acetate and cultured for an additional 6 h. Fatty acids and cholesterol were extracted, and the rates of synthesis were determined. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).
![Figure 2. Chrysin reduces SREBP target gene expression and de novo synthesis of fatty acids and cholesterol.Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a) The cells were then switched to medium C in the presence of either vehicle or the indicated chrysin concentrations. After a 24 h incubation, total RNA was isolated from the cells. Quantitative real-time PCR was performed, and the relative mRNA levels were obtained by normalization to GAPDH. (b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After an 18 h incubation, the cells were treated with 1.6 μCi/mL [Citation14C]acetate and cultured for an additional 6 h. Fatty acids and cholesterol were extracted, and the rates of synthesis were determined. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).](/cms/asset/302f8f00-f392-4591-8ad9-a081fe15da26/tbbb_a_1608806_f0002_b.gif)
Figure 3. Chrysin decreases the protein levels and activity of SREBPs mature forms.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a and b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin (a), or 100 μM chrysin (b). After a 9 h incubation, whole-cell extracts underwent immunoblotting (IB) with anti-SREBP-1 (2A4), anti-SREBP-2 (Rs004) (b), or anti-β-actin antibodies. Similar results were obtained in three separate experiments. (c) Huh-7 cells were transfected with 400 ng of a reporter plasmid, pFAS-luc, together with 200 ng of pCMV-β-gal in the presence or absence of 400 ng of the indicated expression plasmids for mature forms of SREBP-1c or SREBP-2. Twenty-four hours after transfection, the cells were cultured with 1 μg/mL 25-hydroxycholesterol (25HC) or 100 μM chrysin for 24 h. Luciferase assays were performed as described under “materials and methods.” The promoter activities without 25HC and chrysin in the absence of SREBP-1c and −2 are represented as 1. All data are presented as means ± SE (n = 3). (d and e) Huh-7 cells were transfected with pME-18S (Mock), pME-SREBP-1c(1–463), or pME-SREBP-2(1–481) and cultured for 24 h in medium A. The cells were trypsinized, seeded in six well plates, and cultured further for 24 h under the same conditions, after which they were cultured for 24 h with either vehicle or 100 μM chrysin. Whole-cell extracts underwent immunoblotting (IB) with anti-β-actin and anti-SREBP-1 (2A4) (c) or anti-SREBP-2 (1D2) (d) antibodies. Similar results were obtained in three separate experiments.
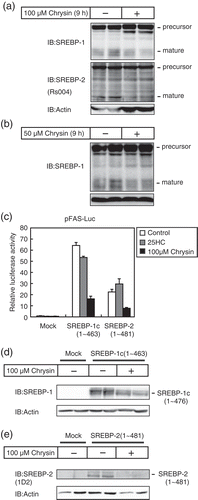
Figure 4. Chrysin-mediated decrease of mature SREBP is not abolished by treatment with the proteasome inhibitor MG132.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. After pre-treatment with 10 μM MG132 for 0.5 h, the cells were incubated for 9 h with medium C supplemented with 10 μM MG132 in the presence of either vehicle or 100 μM chrysin. Whole-cell extracts underwent immunoblotting (IB) with anti-SREBP-1 (2A4) or anti-β-actin antibodies.
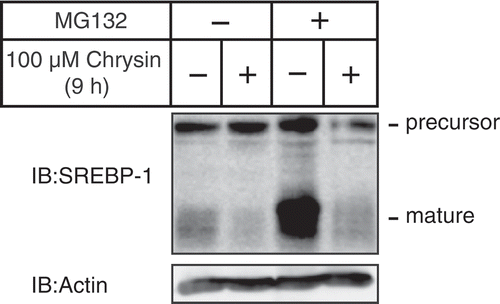
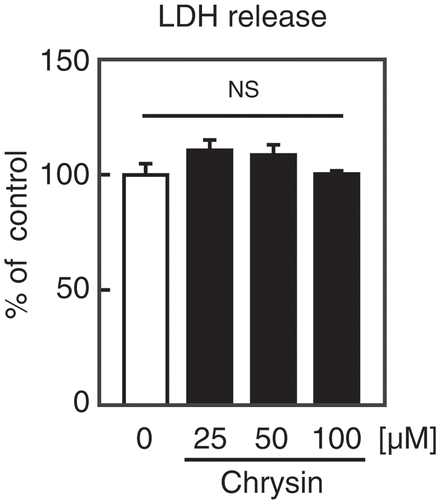
Figure 5. Effect of chrysin on LDH release from Huh-7 cells.
Huh-7 cells were treated with the indicated concentrations of chrysin for 9 h, followed by LDH assay. All data are expressed as means ± SE (n = 4). NS = not significant.