Figures & data
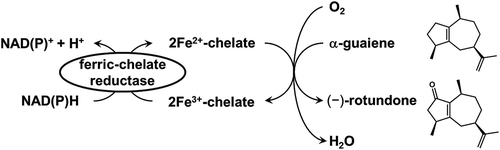
Figure 1. Catalytic synthesis of odor sesquiterpenoid, (−)-rotundone, using non-heme Fe2+-chelate catalyst and ferric-chelate reductase.
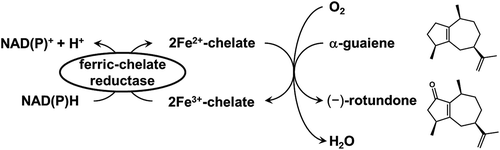
Figure 2. Synthesis of (−)-rotundone using intracellular metal ions. Reaction mixtures contained α-guaiene and 20 mM of each metal salt. The amount of synthesized (−)-rotundone using FeSO4 was defined as 100%.
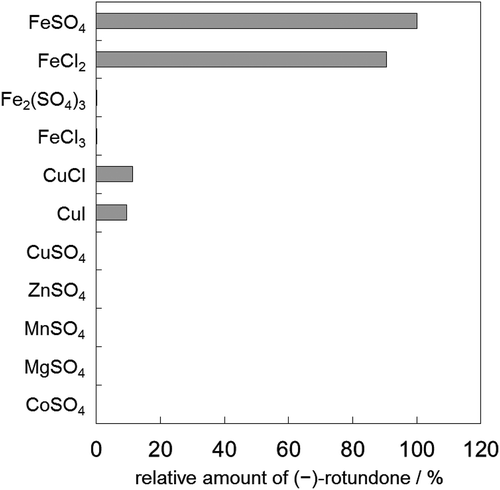
Figure 3. Synthesis of (−)-rotundone using YqjH. (a) Reaction mixtures contained α-guaiene, YqjH, and each cofactor described with a + symbol at 5 mM NAD(P)H or 1 mM Fe(III)-EDTA. (b) Reaction mixtures contained α-guaiene, NADPH, YqjH, and 1 mM of each ferric iron. Data show the means ± standard deviations of three independent experiments.
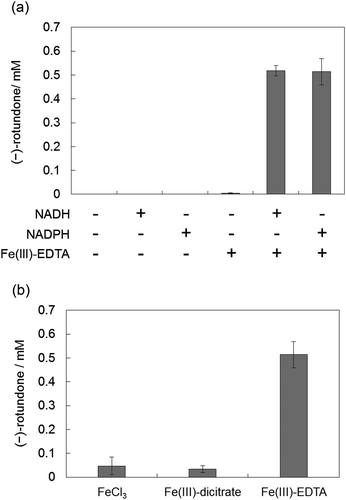
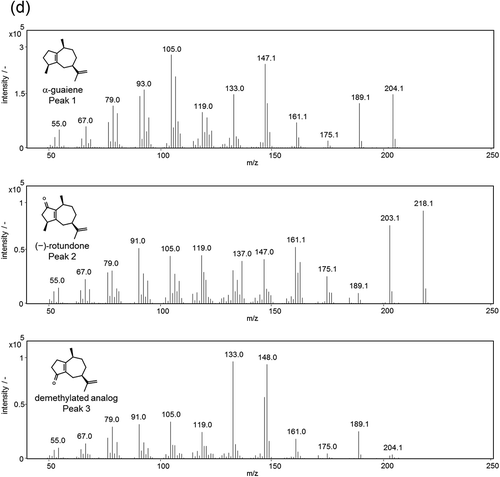
Figure 4. GC-MS analysis of the reaction products containing α-guaiene, NADPH, Fe(III)-EDTA, and YqjH (a), with 100 U/mL catalase (b), or 1 mM galvinoxyl free radical (c). MS spectra of each peak (d); α-guaiene (peak 1), (−)-rotundone (peak 2), and demethylated analog (peak 3).
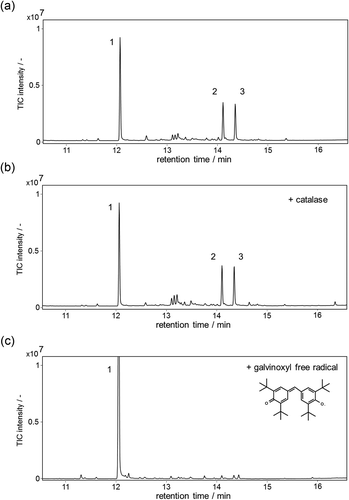
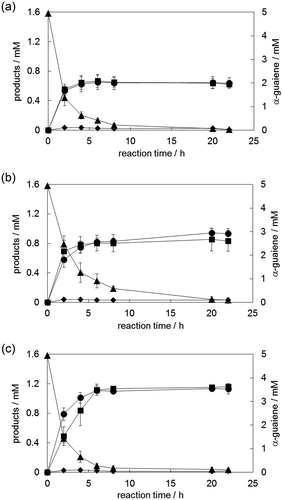
Figure 5. Time courses of (−)-rotundone synthesis using α-guaiene, NADPH, Fe(III)-EDTA, and YqjH (a), with 1% HP-β-CD (b) and 1 mM NAD+, 100 mM glucose, and 0.1 U/mL GlcDH as substitutes for NADPH (c). Circle, (-)-rotundone; square, demethylated analog; diamond, rotundol; triangle, α-guaiene. Data are shown as the means ± standard deviations of three independent experiments.