Figures & data

Figure 1. Purification and characterization of BFP.
(a) Elution profile of polysaccharide fraction extracted from B. fusco-purpurea on a Sephadex G75 column. (b) TLC pattern of BFP developed with n-butanol/glacial acetic acid/water, 2: 2: 1, v/v/v. The first line (a line) is the solvent front, and the second line (b line) is the origin. (c) Profile of BFP in HPLC on a TSK-gel G4000 PWXL column (7.8 mm I.D. × 300 mm) eluted with ultra-pure water (pH = 7.0) at a flow rate of 1.0 mL/min. (d) FT-IR spectrum of BFP.
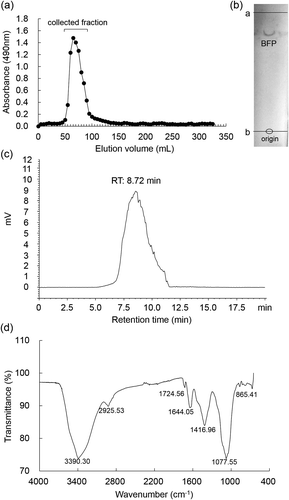
Table 1. Chemical compositions and molecular mass of polysaccharide isolated from B. fusco-purpurea.
Figure 2. Inhibitory effects of BFP on α-amylase and α-glucosidase.
(a) Inhibitory effects of BFP (0–1.0 mg/mL) or acarbose (0.01 mg/mL) on α-amylase. (b) Inhibitory effects of BFP (0–1.0 mg/mL) or acarbose (0.50 mg/mL) on α-glucosidase. All experiments were performed in triplicate, and values are the means ± SD. Asterisks indicate significant differences between with and without polysaccharides (or acarbose). *p <0.05.
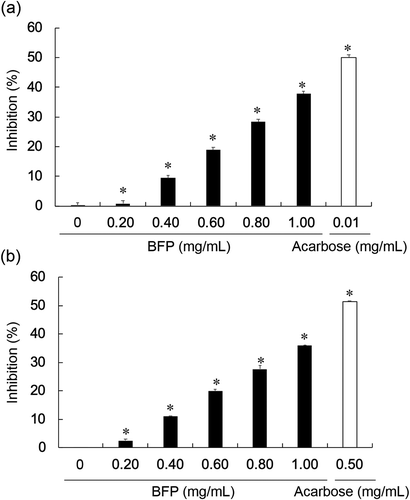
Figure 3. The inhibitory kinetics of BFP on α-amylase and α-glucosidase.
(a) Linear regression of reaction rates versus different concentrations of α-amylase in the presence (closed circles) and absence (open circles) of BFP. The final concentration of starch was 5.0 mg/mL, and the reaction time was 3 min. (b) Linear regression curves of reaction rates versus different concentrations of α-glucosidase in the presence (closed circles) and absence (open circles) of BFP. The final concentration of pNPG was 2.5 mM, and the reaction time was 3 min. (c) Lineweaver–Burk plot of the reactions of α-amylase in the presence (closed circles) and absence (open circles) of BFP. (d) Lineweaver–Burk plot of the reactions of α-amylase in the presence (closed circles) and absence (open circles) of BFP. All experiments were performed in triplicate, and values were the means ± SD.
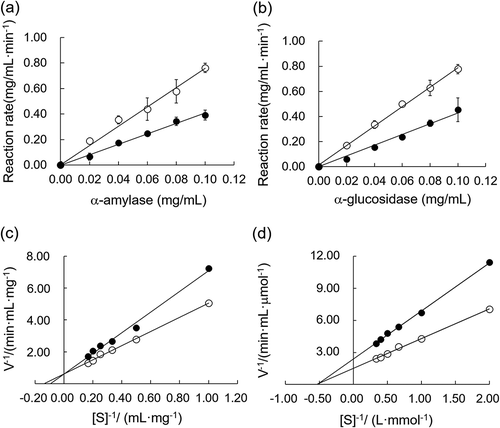
Table 2. Effects of BFP on the secondary structures of α-amylase and α-glucosidase.
Figure 4. Effects of BFP on the CD spectrum of α-amylase or α-glucosidase.
CD spectra of α-amylase (a) or α-glucosidase at 5.0 mg/mL (b) in the presence (dotted line) or absence (solid line) of BFP (1000 µg/mL) were measured. Measurements were carried out after 15 min incubation of the mixture of enzyme and BFP at 25°C.
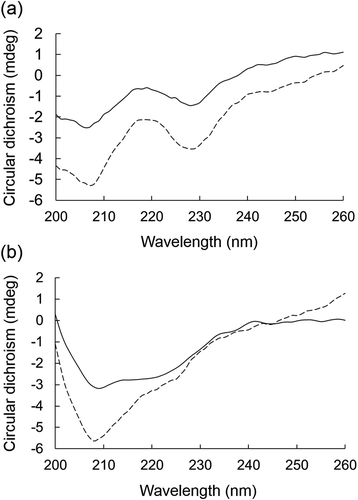
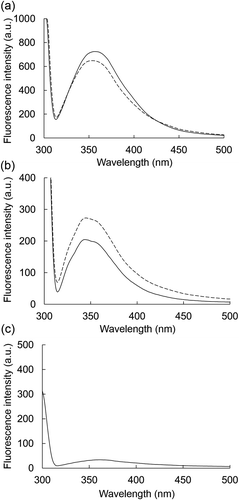
Figure 5. Effects of BFP on the fluorescence spectrum of α-amylase or α-glucosidase.
Fluorescence emission spectra of α-amylase (a) or α-glucosidase (b) at 1.0 mg/mL in the presence (dotted line) or absence (solid line) of BFP (1000 µg/mL) were measured. Measurements were carried out after 15 min incubation of the mixture of enzyme and BFP at 25°C. Fluorescence emission spectrum of BFP alone (c) at 1000 µg/mL.