Figures & data
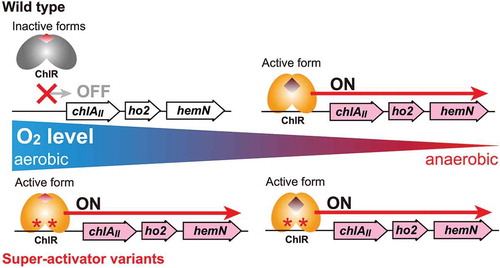
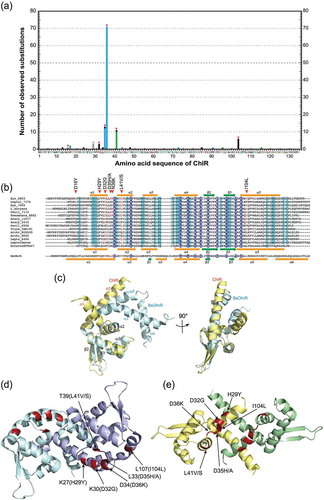
Figure 1. Chlorophyll, heme, and bilin biosynthesis pathways (a) and complementation of ∆chlAI with overexpression of chlAII (b).
(a) Chlorophyll, heme, and bilin biosynthesis pathways. In cyanobacteria, all tetrapyrrole pigments are produced from glutamate and shared with the common pathway from glutamate to protoporphyrin IX (PPN). Two biosynthetic pathways toward chlorophyll (Mg-branch) and heme/bilin (Fe-branch) diverge from PPN. Three reactions catalyzed by two different enzymes, aerobic and low-oxygen/anaerobic enzymes, are shown blue and red arrows, respectively. Coproporphyrinogen III oxidase in the common pathway is catalyzed by HemF (aerobic) and HemN (anaerobic). 3,8-divinyl protochlorophyllide formation from Mg-PPN monomethyester cyclase in the Mg-branch is catalyzed by two ChlA isoforms, ChlAI (aerobic) and ChlAII (low-oxygen). Biliverdin IXα formation from protoheme is catalyzed by two HO isoforms, HO1 (aerobic) and HO2 (low-oxygen), in the Fe-branch. (b) Photoautotrophic growth of WT, ∆chlAI, ΔchlAI mutants overexpressing ChlAI (ΔchlAI/A1-ox), and ChlAII (ΔchlAI/A2-ox). ∆chlAI/A1-ox, ∆chlAI/A2-ox, WT, and ΔchlAI cells were all grown under aerobic and low-oxygen conditions for 4 days. Spots 1, 4; 2, 5; and 3, 6 indicate that the initial cell densities were 1.0, 0.1 and 0.01 at OD730, respectively. Spots 1 to 3 and 4 to 6 were incubated under aerobic and low oxygen conditions, respectively. Gene arrangements of ∆chlAI/A1-ox and ∆chlAI/A2-ox at the genome neutral site (slr2030-slr2031) are shown (left). chlAI and chlAII were ectopically overexpressed under the trc promoter in ∆chlAI/A1-ox and ∆chlAI/A2-ox.
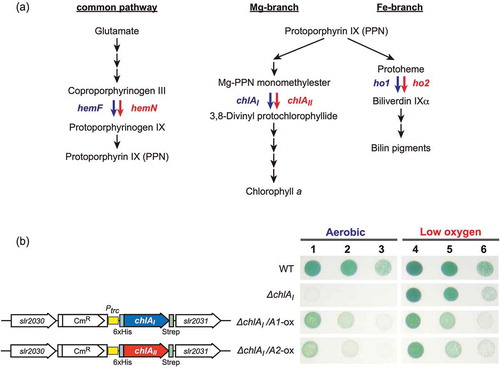
Figure 2. Isolation of pseudorevertants of ∆chlAI by transformation with the epPCR-amplified chlR gene fragments.
(a) Gene arrangement of chlR (slr1598-sll1512-sll1513) in the genome of Synechocystis 6803. To prepare DNA fragments to isolate pseudorevertants carrying super-activator mutations in chlR, a 2.3 kb fragment was amplified by epPCR (shown as a thick orange bar). Arrows indicate primers (sll1512-f2 and sll1512-r2 [Citation11]) used in epPCR. (b) Colonies of pseudorevertants appeared on an agar plate after transformation of ∆chlAI with epPCR DNA fragments (left). No colonies appeared without epPCR DNA fragments (right). (c) Photoautotrophic growth of pseudorevertants. Pseudorevertants were incubated under low-oxygen (lane 1) and aerobic (lane 2) conditions as were WT and ∆chlAI cells.
![Figure 2. Isolation of pseudorevertants of ∆chlAI by transformation with the epPCR-amplified chlR gene fragments.(a) Gene arrangement of chlR (slr1598-sll1512-sll1513) in the genome of Synechocystis 6803. To prepare DNA fragments to isolate pseudorevertants carrying super-activator mutations in chlR, a 2.3 kb fragment was amplified by epPCR (shown as a thick orange bar). Arrows indicate primers (sll1512-f2 and sll1512-r2 [Citation11]) used in epPCR. (b) Colonies of pseudorevertants appeared on an agar plate after transformation of ∆chlAI with epPCR DNA fragments (left). No colonies appeared without epPCR DNA fragments (right). (c) Photoautotrophic growth of pseudorevertants. Pseudorevertants were incubated under low-oxygen (lane 1) and aerobic (lane 2) conditions as were WT and ∆chlAI cells.](/cms/asset/b462fc8a-a668-4a20-8211-2d503fe1fddb/tbbb_a_1687281_f0002_oc.jpg)
Table 1. List of mutations in the chlR gene in pseudorevertants from ∆chlAI.
Figure 3. Transcript levels of chlAII (a) and chlR (b) in the pseudorevertants. Transcript levels of chlAII and chlR in pseudorevertant cells grown under aerobic (blue) and low oxygen (pink) conditions were determined by real-time PCR. Relative transcript levels to those of rrn16 in the wild type grown under aerobic conditions were set to 1.0. Bars are SDs in technical triplicates. The relative transcript levels are shown on a logarithmic scale and a linear scale for chlAII (a) and chlR (b), respectively.
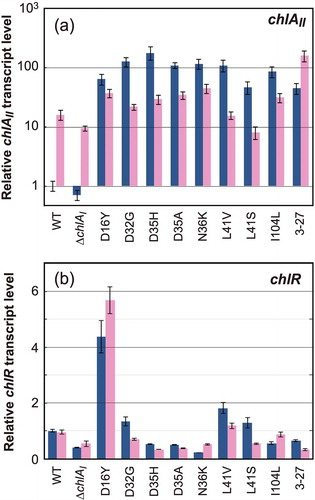
Figure 4. Mapping of the amino acid residues responsible for super-activator variants of ChlR.
(a) ChlR super-activator substitutions in pseudorevertants generated by transformation with epPCR chlR fragments are plotted against the horizontal axis of the ChlR amino acid sequence. Red asterisks indicate amino acid residues that turn ChlR into a super-activator form via a single substitution. H29 indicated with a gray asterisk is accompanied with other substitutions in ChlR. Color of bars and amino acid residues in the horizontal axis indicate property of the substituted amino acid residues; red, acidic (D and E); blue, basic (H, K, and R); gray, aromatic (F, W, and Y); green, polar (C, N, Q, S, and T); and black, nonpolar (A, G, I, L, M, P, and V). (b) Multiple alignments of ChlR amino acid sequences, 13 probable ChlR orthologs from other cyanobacteria, and EF0647 from E. feacalis V583, and OhrR (B.subtilis). Secondary structure of EF0647, OhrR, and predicted ChlR structure are shown: orange bars, α helices 1–6; green arrows, β sheets 1–3. In the structures of EF0647 and ChlR the β1 sheet is missing but for consistency in the names of β2 and β3 sheets are adopted. Syn_6803, Synechocystis sp. PCC 6803; Leptol_7376, Leptolyngbya sp. PCC 7376; Syn_7002, Synechococcus sp. PCC 7002; L_boryana, Leptolyngbya boryana dg5; Oscil_7112, Oscillatoria nigro-viridis PCC 7112; Psanab_6802, Pseudoanabaena sp. PCC 6802; Acaryoc_MBIC11017, Acaryochloris marina MBIC11017; Acaryoc_CCMEE5410, Acaryochloris marina CCMEE5410; Oscil_6506, Oscillatoria sp. PCC 6506; Tricho_IMS101, Trichodesmium erythraeum IMS101; Arthr_NIES-39, Arthrospira platensis NIES-39; Arthr_8005, Arthrospira sp. PCC 8005; Leptol_6406, Leptolyngbya sp. PCC 6406; LeptolHoron; Leptolyngbya sp. Heron Island J; EnterocEF0647, E. feacalis V583; and BsOhrR, B. subtilis. (c) Superposition of ChlR putative model (light yellow) and the BsOhrR crystal structure (light blue) in the protomer. (d, e) Structural comparison between OhrR (d) and the ChlR putative dimeric model (e) in dimeric state. Six amino acid residues of OhrR (K27, K30, L33, D34, T39, and L107) corresponding to H29, D32, D35, D36, L41 and I104 of ChlR, respectively, are shown in red as the amino acid residues in the OhrR protein (d). The six amino acid residues responsible for the super-activation variants are shown in red (e).