Figures & data
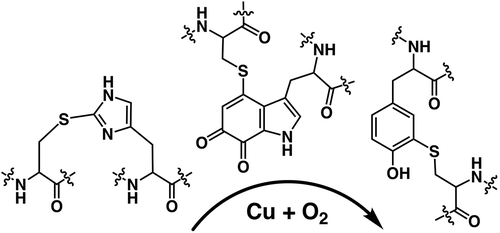
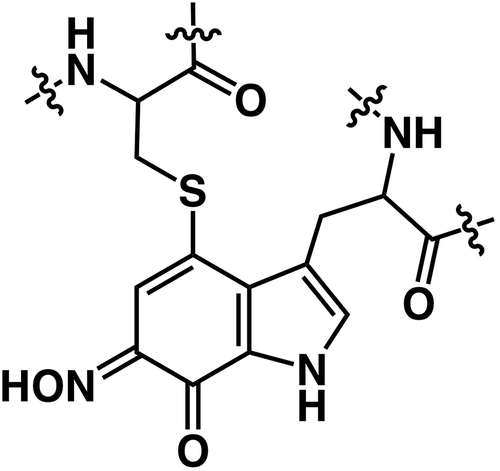
Figure 1. The structural representation of characteristic amino acids generated by the post-translational modifications from heteroaromatic amino acid side chains.
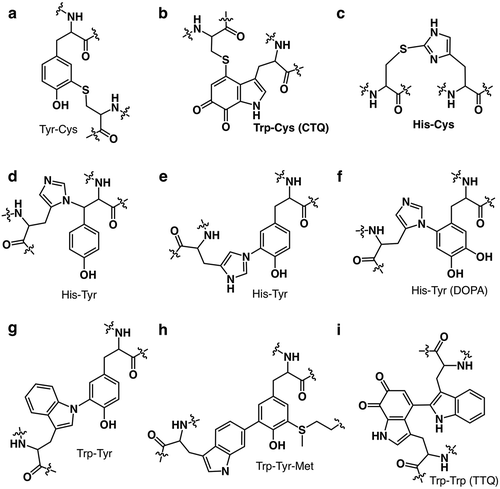
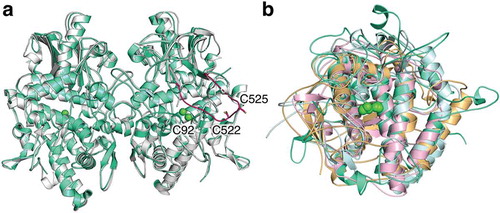
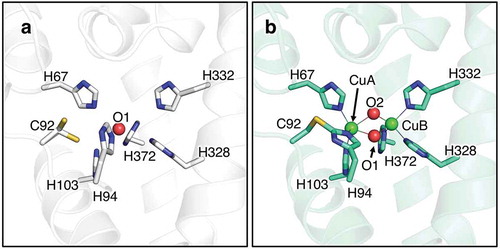
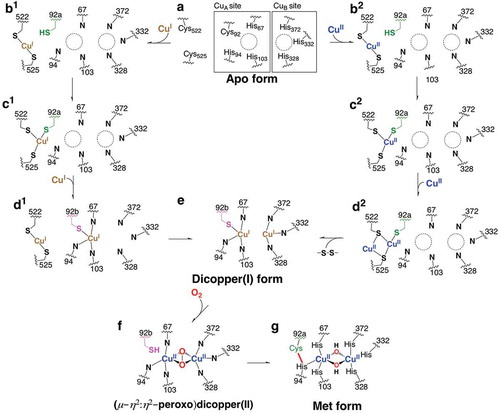
Figure 2. The superimposed crystal structure of melB pro-tyrosinase with other tyrosinases. (a) whole structure of apo (white, PDB code:3W6Q) and holo form (green, PDB code:3W6W) (b) Copper-binding domains of mushroom (blue, PDB code: 2y9w), plant catechol oxidase (orange, PDB code: 1BT3), and the functional unit g of octopus hemocyanin (pink, PDB code: 1JS8). The protein main chain is displayed as ribbon and key cysteine residues are highlighted as sticks and green sphere indicates copper.