Figures & data
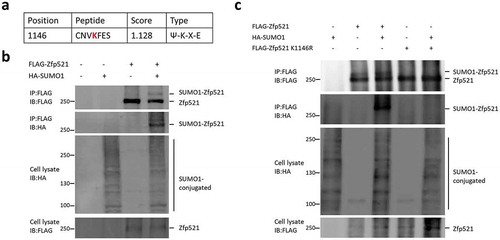
Table 1. Sequences of guide RNA, donor oligo, PCR genotyping primer and DNA sequencing primer.
Figure 1. Zfp521 was modified by SUMO1 on lysine 1146. (a) Potential SUMO modification site in Zfp521 was predicted by SUMOsp 2.0 software. (b) Zfp521 SUMOylation status was measured by immunoprecipitation (IP) and immunoblotting (IB) after co-transfection of HEK293T cells with HA-SUMO1 and FLAG-Zfp521. (c) Zfp521 SUMOylation status was detected by IP and IB after co-transfection of HEK293T cells with HA-SUMO1, FLAG-Zfp521 or Zfp521-K1146R mutant.
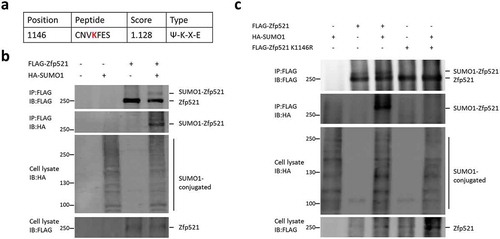
Figure 2. Zfp521 SUMOylation-deficient mice were generated. (a) Schematic representation of the gene structure of Zfp521. The primary SUMOylation site of lysine 1146 was mutated to arginine (K1146R). (b) The genotypes of mice were determined by PCR. (c) The genotypes of Zfp521 wild-type (Zfp521WT), K1146R heterozygote mutant (Zfp521±) and homozygous mutant (Zfp521mutant) mice were confirmed by DNA Sequence. (d) Zfp521 mRNA levels were detected by RT-qPCR in peripheral blood (PB) and bone marrow (BM) of Zfp521WT and Zfp521mutant mice at 7 weeks of age, expressed as percent of expression in Zfp521WT mice (n = 3 per group). (e) Zfp521 protein levels were detected by western blotting in PB and BM of Zfp521mutant and Zfp521WT mice at 7 weeks of age.
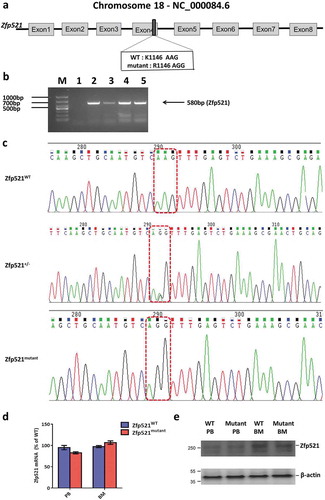
Figure 3. Zfp521 SUMOylation-deficient mice had normal peripheral blood counts. (a and b) Absolute number of white blood cells (WBC), neutrophils (NE), lymphocytes (LY), monocytes (MO), eosinophils (EO), basophils (BA) (a), platelets (PLT), red blood cells (RBC), and hemoglobin (HB) (b) in peripheral blood of Zfp521mutant and Zfp521WT mice at 7 weeks of age. Data were from two independent experiments (n = 4, per experiment) and present as mean ± SD.
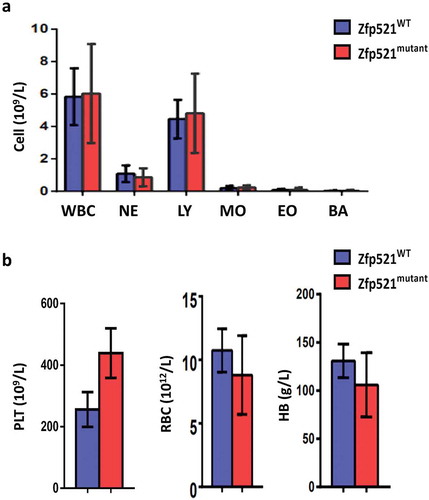
Figure 4. Zfp521 SUMOylation-deficient mice had normal mature blood cells in bone marrow, spleen and thymus. (a-c) Flow cytometric analysis and absolute number of different development stages of erythrocytes (a), myeloid cells (b), and B cells (c) in bone marrow (BM) and spleen of Zfp521mutant and Zfp521WT mice at 7 weeks of age. (d) Flow cytometric analysis and absolute numbers of T cells in BM, spleen, and thymus of Zfp521mutant and Zfp521WT mice at 7 weeks of age. Data were from two independent experiments (n = 4, per experiment) and present as mean ± SD.
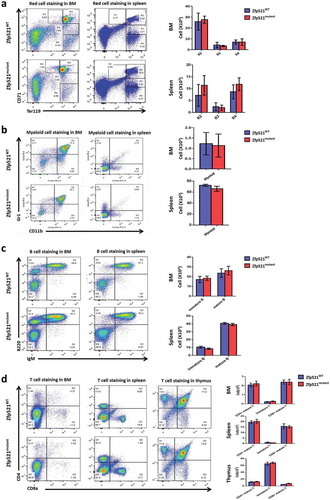
Figure 5. Zfp521 SUMOylation-deficient mice had normal primitive cells in bone marrow. (a-c) Flow cytometry analysis and absolute number of hematopoietic stem cells (HSC), hematopoietic progenitor cells (HPC) and LSK cells (a), and granulocyte-macrophage progenitors (GMP), megakaryocyte-erythroid progenitors (MEP) and common myeloid progenitors (CMP) (b), and lympho-primed pluripotent progenitor cells (LMPP) in BM of Zfp521mutant and Zfp521WT mice at 7 weeks of age. Data were from two independent experiments (n = 4, per experiment) and present as mean ± SD.
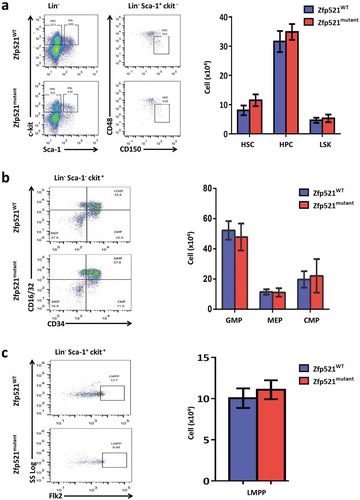
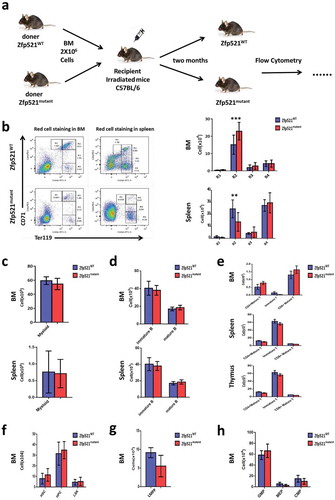
Figure 6. Erythroid hematopoietic recovery of transplanted mice was affected by Zfp521 SUMOylation. (a) The diagram for the experimental design. (b) Flow cytometric analysis and absolute number of different development stages of erythrocytes in BM and spleen of 8-week old recipient mice after transplantation BM cells from Zfp521mutant mice and Zfp521WT mice. (c and d) Absolute number of myeloid cells (c) and B cells (d) in BM and spleen of 8-week old recipient mice after transplantation BM cells from Zfp521mutant mice and Zfp521WT mice. (e) Absolute number of T cells in the BM, spleen, and thymus of 8-week old recipient mice after transplantation BM cells from Zfp521mutant mice and Zfp521WT mice. (f-h) Absolute number of HPC, LSK and HSC cells (f), LMPP cells (g), and CMP, GMP and MEP cells (h) in BM of 8-week old recipient mice after transplantation BM cells from Zfp521mutant mice and Zfp521WT mice. Data were from three independent experiments (n = 3, per experiment) and present as mean ± SD. (***p < 0.001).