Figures & data
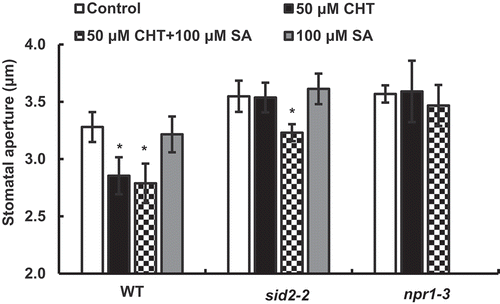
Figure 1. Chitosan (CHT)-induced responses of stomatal apertures in Arabidopsis wild type (WT) and sid2-2 mutant. (a) CHT (50 µg/mL) induced stomatal closure in WT but not in the sid2-2 plants. (b) Stomatal closure induced by co-treatment with CHT (50 µg/mL) and SA (100 µM) in WT and the sid2-2 plants. (c) SA at 50 µM and 100 µM did not induce stomatal closure in WT and the sid2-2 plants. Each bar shows average of stomatal apertures of 80 stomata (n = 4, where each experiment is from 20 stomata data). Error bars represent SE. * indicates significant at p < 0.05.
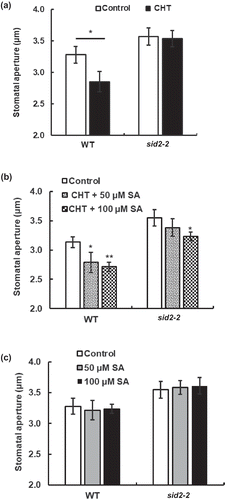
Figure 2. Chitosan (CHT)-elicited apoplastic ROS production in wild type (WT) and sid2-2 mutant. (a), CHT (50 µg/mL) induced apoplastic ROS production in WT but not in the sid2-2 mutant. (b) Apoplastic ROS production induced by co-treatment with CHT (50 µg/mL) and SA (100 µM) in WT and the sid2-2 plants. (c) SA at 100 µM did not induce apoplastic ROS production both in WT and the sid2-2 plants. Each bar shows averages of ROS production from 16 leaves (n = 4). The vertical scale shows the relative values of pixel intensity of the DAB brown color of the H2O2 formation when the values of leaves treated with CHT, SA, and both CHT and SA are normalized to control value taken as 100 for each experiment. Error bars stand for SE. * indicates significant at p < 0.05.
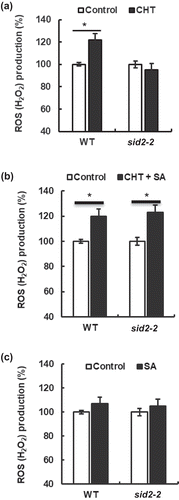
Figure 3. Impairment of chitosan (CHT)-induced stomatal closure in NPR1-disruption mutant npr1-3. (a) CHT at 50 µg/mL induced stomatal closure in WT, but not in the npr1-3 in the absence of SA. (b) 50 µg/mL CHT in the presence of 100 µM SA did not induce stomatal closure in the npr1-3 mutant. Each bar represents averages of stomatal apertures from at least total 60 stomata. Stomatal apertures from three independent experiments (n = 3) are shown. Error bars stand for SE. * indicates significant at p < 0.05.
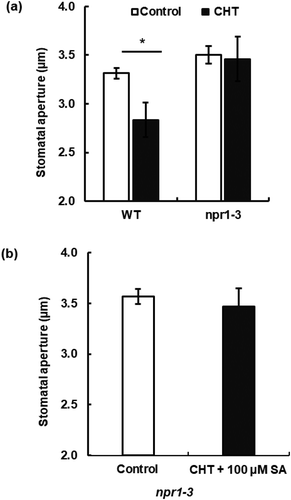
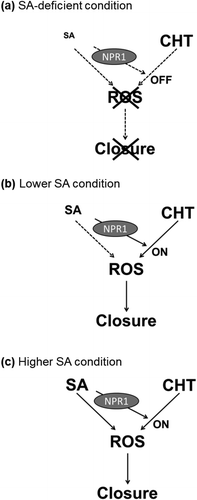
Figure 4. A scheme of interaction between chitosan (CHT) signaling and salicylic acid (SA) signaling in guard cells. (a) Salicylic acid (SA)-deficient condition; the chitosan signal pathway is not activated by SA. Consequently, chitosan cannot induce stomatal closure. (b) Lower SA condition; the chitosan signal pathway is activated by SA, which is mediated by NPR1. As a consequence, chitosan induces ROS production, resulting in stomatal closure. (c) Higher SA condition; SA activates both the SA signal pathway and the chitosan signal pathway. As a result, SA induces stomatal closure.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
