Figures & data
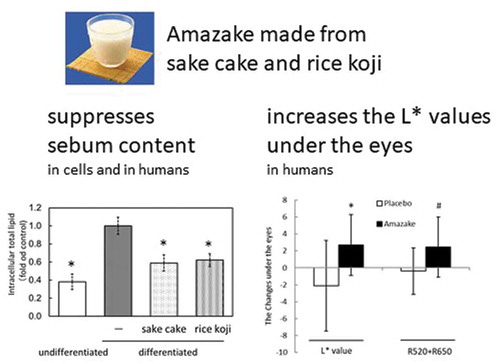
Table 1. Subject background information.
Table 2. Composition of the freeze-dried amazake-like placebo and amazake.
Figure 1. Lipid metabolism assay using hamster sebocytes. Quantification of intracellular total lipids (a), PPARα mRNA (b), and PPARγ mRNA (c). Data are shown as mean ± standard deviation. Intracellular total lipids of undifferentiated or differentiated hamster sebocytes without treatment or treated with sake cake or rice koji were measured using Oil Red O. Relative gene expression of PPARα and PPARγ of undifferentiated or differentiated hamster sebocytes without treatment or treated with sake cake or rice koji were investigated using qRT-PCR. *; P < 0.05, significant difference compared to control differentiated cells (untreated).
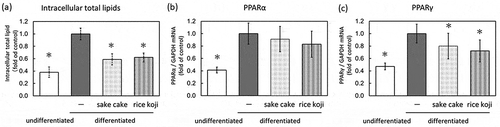
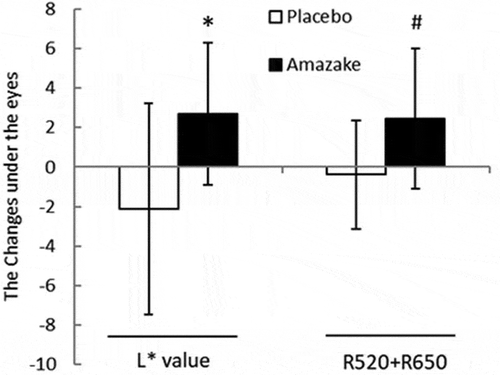
Figure 2. Skin measurements of the placebo (Placebo) and amazake groups (Amazake). Sebum content (a), skin barrier: transepidermal water loss (b), skin viscoelasticity: R0 (c), R2 (d), R7 (e), skin color: R520+ R650 (f), L* (g), a (h), b (i), and skin temperature (j). Data are shown as mean ± standard deviation. Skin conditions of subjects who ingested placebo or amazake were measured using specialized equipment at 0 and 4 weeks. *; intra-group P < 0.05, significant difference between 0 and 4 weeks. #; inter-group P < 0.05, significant difference between Placebo and Amazake.
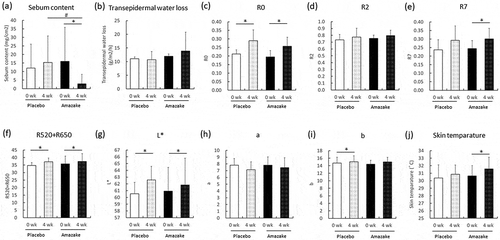
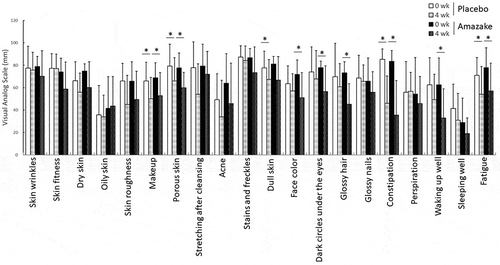
Figure 3. Differences in visual analog scale indices for specified conditions. Data are shown as mean ± standard deviation. Questionnaires were undertaken by subjects who ingested placebo (Placebo) or amazake (Amazake) at 0 and 4 weeks. *; Intra-group P < 0.05, significant difference between 0 and 4 weeks.