Figures & data
TABLE 1. Criteria for treatment failure for VISUAL I and VISUAL II clinical trials.
TABLE 2. Primary endpoint and ranked secondary endpoints for VISUAL I and VISUAL II trials.
TABLE 3. Participants’ demographics and baseline characteristics (intent-to-treat population).
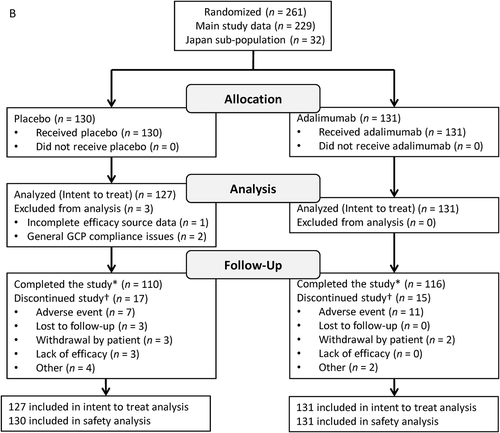
FIGURE 1. Participant disposition. VISUAL I (A) and VISUAL II (B). GCP = Good Clinical Practice.
*Includes participants who experienced treatment failure, reached 80 weeks of treatment without treatment failure, or had to terminate the study because the planned number of treatment failures was reached.†Some participants had multiple reasons for discontinuation; total counts for reasons for discontinuation exceed the total number of discontinuations.
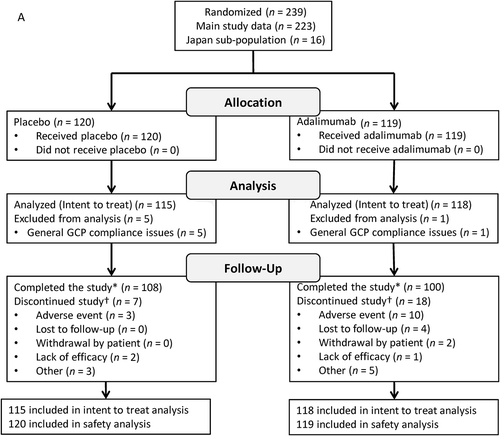
TABLE 4. Primary endpoint for main study, integrated study, and Japan substudy for VISUAL I and VISUAL II trials.
TABLE 5. Summary of ranked secondary efficacy variables*.
TABLE 6. Summary of adverse events (safety population).