Figures & data
Figure 1. Optical coherence tomography of a patient with IOI involving the posterior segment of the eye. Typical changes strongly suggestive of IOI include hyperreflective spots in the pre-retinal vitreous, an irregular inner retinal surface (arrows) and hyperreflective spots in all retinal layers without significant structural damage to the neuroretina as long as the macula is not affected by vascular occlusion. Typically, the anatomic effect of the drug with reduction of retinal fluid is also visible compared to the pre-injection OCT.
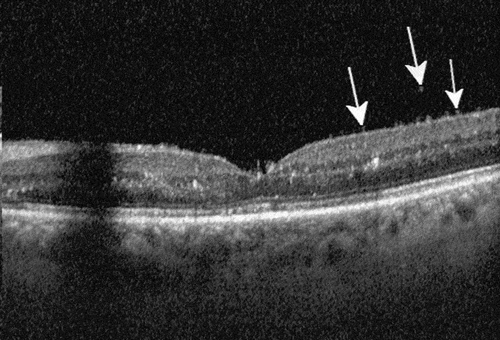
Figure 2. A. Mild anterior uveitis with discrete vision loss and mutton fat precipitates on the corneal endothelium six weeks after the second Brolucizumab injection which was well controlled under topical corticosteroids.B. Same patient, one week later. The posterior segment looks rather quiet; bleedings, vasculitic changes or vascular occlusion are not obvious despite a diffuse cellular vitreal infiltration. The dark arrow points to a small retinal vessel without obvious incoherence. C. Same patient, same visit as B.
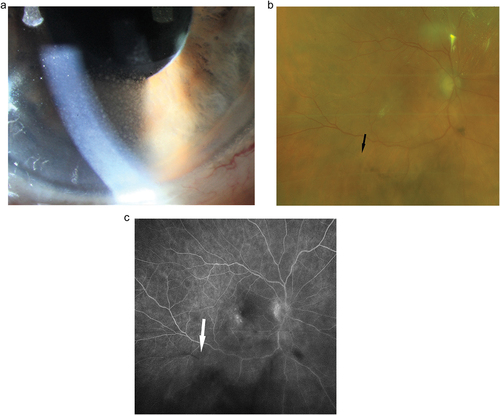
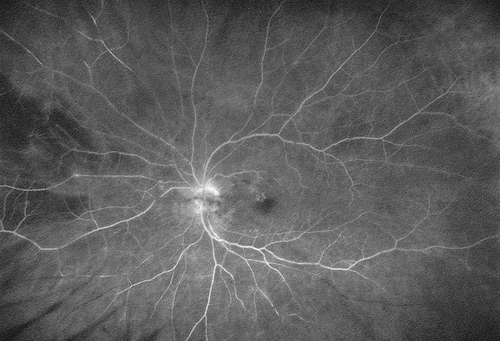
Figure 3. Eighty-four years old male presenting with foggy vision four weeks after his third Brolucizumab injection. Fluorescein angiography reveals a clinically not visible mild vasculitis and multiple vascular occlusions in the nasal and temporal inferior vessel arcades. In the absence of macular involvement, visual acuity fully recovered under intravenous corticosteroids.