Figures & data
Figure 1. A two-step model has been proposed to explain the initiation of NLRP3 inflammasome activation. The first step entails system priming through the NF-κB signalling pathway, promoting the transcription of NLRP3, pro-IL-1β, and pro-IL-18. In the second step, the inflammasome activates upon the recognition of PAMPS or DAMPS. NLRP3 associates with the adaptor protein ASC, prompting the recruitment of procaspase-1, which leads to caspase-1 self-cleaving activation. The ensuing inflammatory response significantly hinges on caspase-1, facilitating the conversion of inactive pro-IL-1β and pro-IL-18 into active IL-1β and IL-18. Caspase activation also triggers pyroptosis; a specific form of programmed cell death. The red boxes depict the sites within inflammasome activation, where pathogenic variants drive the development of monogenic autoinflammatory syndromes; ASC: Apoptosis-Associated Speck-Like Protein, BS: Blau Syndrome, CAPS: Cryopyrin-Associated Periodic Syndromes, DAMPs: Damage-Associated Molecular Patterns, FMF: Familial Mediterranean Fever, HA20: Haploinsufficiency A20, IL: Interleukin, LPS: Lipopolysaccharide. NLRP3: nucleotide-binding leucine-rich repeat-containing receptor 3, NOD2: Nucleotide-Binding Oligomerisation Domain-Containing Protein 2, NF-κB: Nuclear Factor Kappa B, PAMPs: Pathogen-Associated Molecular Patterns, ROSAH: Retinal dystrophy, Optic nerve oedema, Splenomegaly, Anhidrosis, and Headache Syndrome, TLR4: Toll-Like Receptor 4, TNFα: Tumour Necrosis Factor Alpha, TNFR1: Tumour Necrosis Factor Receptor 1, TRAPS: Tumour Necrosis Factor Receptor-Associated Periodic Syndrome.
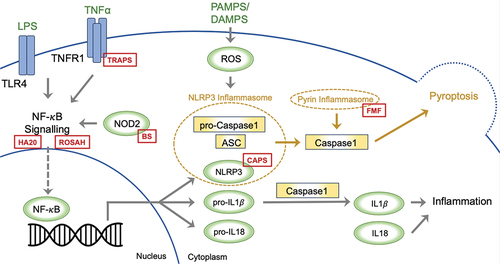
Table 1. Monogenic autoinflammatory syndromes clinical features.
Table 2. Affected pathways and suggested treatments. Biologics are increasingly used upfront alongside non-specific targets such as steroids.
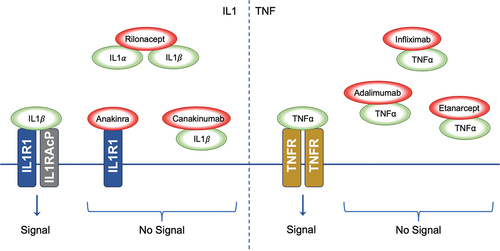
Figure 2. Inhibition of Cytokine Signalling by Therapeutic Agents. The left panel demonstrates the signalling cascade initiated by IL1 binding to the IL1R1-IL1RACP complex. IL1α and IL1β interaction with their receptor results in downstream signalling. The biologic agents Anakinra, Rilonacept, and Canakinumab disrupt IL1 signalling; Anakinra by antagonising receptor interaction, Rilonacept by sequestering IL1α and IL1β, and Canakinumab by neutralising IL1β, thus inhibiting the signal. The right panel shows TNFα signalling through the TNFR. The biologics Infliximab, Adalimumab, and Etanercept impede TNFα interaction with TNFR, thereby preventing signal transduction; IL1: Interleukin-1, IL1α: Interleukin-1 alpha, IL1β: Interleukin-1 beta, IL1R1: Interleukin-1 Receptor Type 1, IL1RACP: Interleukin-1 Receptor Accessory Protein, TNFα: Tumour Necrosis Factor-alpha, TNFR: Tumour Necrosis Factor Receptor.