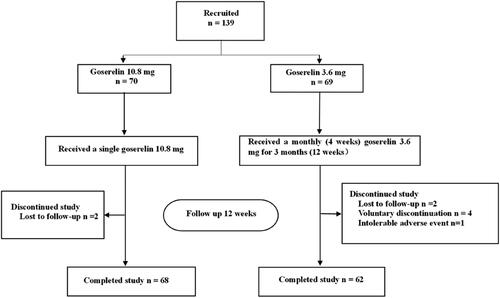
Figures & data
Table 1. Baseline patient demographics and disease characteristics.
Figure 2. Dysmenorrhea scores from baseline to 12 weeks in goserelin 10.8 mg and 3.6 mg groups, 12 weeks vs baseline p < .0001.
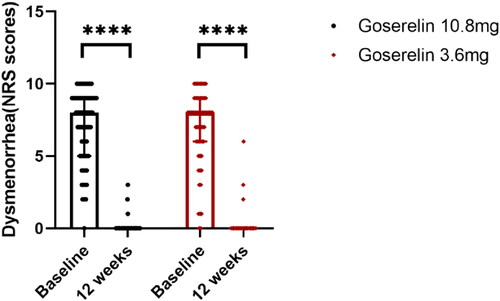
Figure 3. Uterine volume from baseline to 12 weeks in goserelin 10.8 mg and 3.6 mg groups, 12 weeks vs baseline p < .0001.
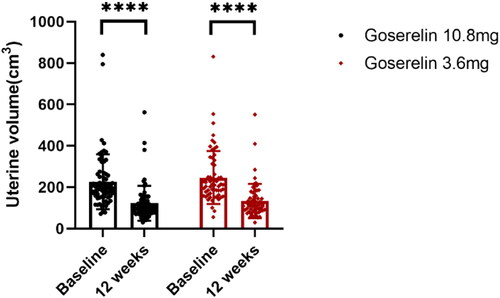
Figure 4. CA125 from baseline to 12 weeks in goserelin 10.8 mg and 3.6 mg groups, 12 weeks vs baseline p < .0001.
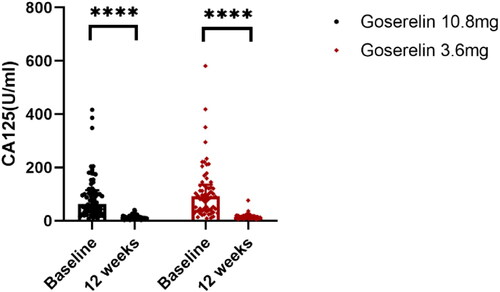
Figure 5. HGB from baseline to 12 weeks in goserelin 10.8 mg and 3.6 mg groups, 12 weeks vs baseline p < .0001.
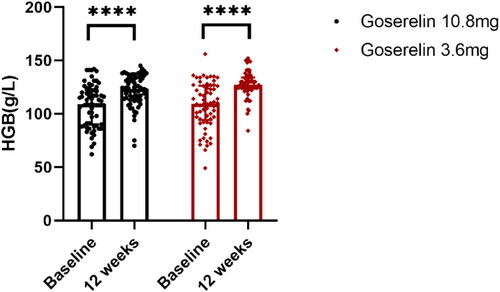
Table 2. Treatment outcomes in goserelin 10.8 mg and goserelin 3.6 mg groups.
Table 3. Incidence of adverse events occurring in goserelin 10.8 mg and 3.6 mg groups.