Figures & data
Table 1. Demographics and baseline characteristics of European women at baseline.
Figure 1. (A) Proportion of European women with reduction in MBL (treatment responders)a at week 24 and (B) percentage change in MBL volume in European women from baseline to week 24/EOT. **Nominal p < .0001 vs. placebo. Error bars show 95% confidence intervals. (A) p Values assessed using the Cochran–Mantel–Haenszel test for proportions stratified by baseline MBL volume. aTreatment responders were defined as women who achieved a MBL <80 mL and a ≥50% reduction from baseline in MBL volume over the last 35 days of treatment. Nominal p value based on pooled data not adjusted for multiplicity. MBL volume is calculated as sum of menstrual blood loss volume from all feminine products assessed by the alkaline hematin method at week 24/end of treatment visit. (B) LS means and 95% confidence intervals for MBL volume are from mixed model with visit, treatment and an interaction term of visit and treatment as fixed effect. The multiple visits for each patient were the repeated measures as random effect within each patient and an autoregressive covariance. Error bars show 95% confidence intervals. The lower limit of the 95% confidence interval was truncated to –100 in instances where values fell below –100%. CT: combination therapy; EOT: end of treatment; LS: least squares; MBL: menstrual blood loss.
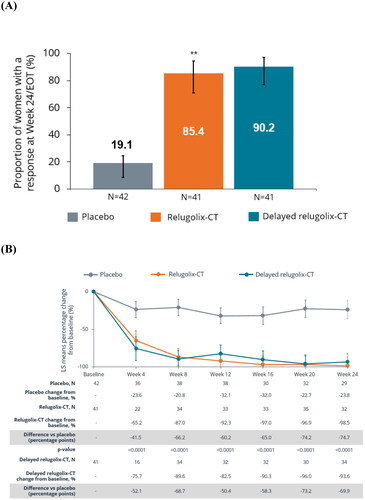
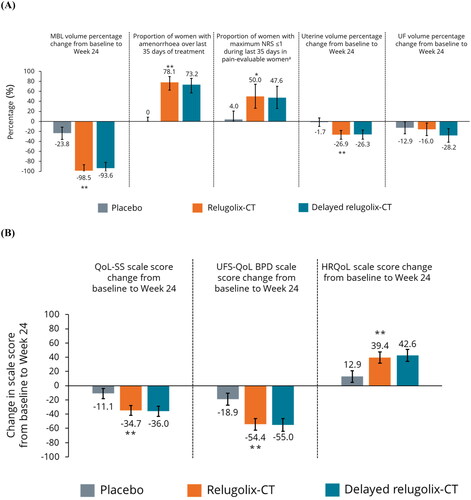
Figure 2. Secondary endpoints for efficacy (A) and QoL (B) in European women. *Nominal p < .001 vs. placebo; **nominal p < .0001 vs. placebo. Error bars show 95% confidence intervals. p Values for categorical outcomes are from the Cochran–Mantel–Haenszel test stratified by MBL volume. p Values (and LS means and 95% confidence intervals) for MBL are from mixed model with visit, treatment and an interaction term of visit and treatment as fixed effect. The multiple visits for each patient were the repeated measures as random effect within each patient and an autoregressive covariance. p Values (and LS means and 95% confidence intervals) for uterine volume change and UF volume change from baseline to week 24 are from generalized linear model with treatment and baseline value of uterine volume or UF volume as covariates. p Values (and LS means and 95% confidence intervals) for QoL-SS, UFS-QoL, and HRQoL are based on a mixed-effect model with treatment, visit, baseline MBL volume and treatment by visit interaction included as fixed effects. The multiple visits for each patient were the repeated measures as random effect within each patient and an unstructured covariance. The lower limit of the 95% confidence interval was truncated to –100 in instances where values fell below –100%. aA smaller number of women were included in the pain-evaluable population for each treatment arm: placebo, N = 25; relugolix-CT, N = 18; delayed relugolix-CT, N = 21. BPD: Bleeding and Pelvic Discomfort; CT: combination therapy; HRQoL: health-related quality of life; LS: least squares; MBL: menstrual blood loss; NRS: Numerical Rating Scale; QoL: quality of life; SS: symptom severity; UF: uterine fibroids; UFS-QoL: uterine fibroid symptom and quality of life.
Supplemental Material
Download MS Word (548.6 KB)Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
