Figures & data
Figure 1. Negative association between ERRγ and VEGFA expression in human endometrial cancer tissue specimens. A–B, Representative immunohistochemical staining for ERRγ in EC tissues with low (A) and high (B) intensity. C–D, Immunohistochemical staining for VEGFA in the same areas of ERRγ (Original magnification: 400 × for the inserts, 100 × for all others; scale bar, 50 μm). E, Histological scores of ERRγ and VEGFA in the 23 EC specimens. ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; EC, endometrial cancer.
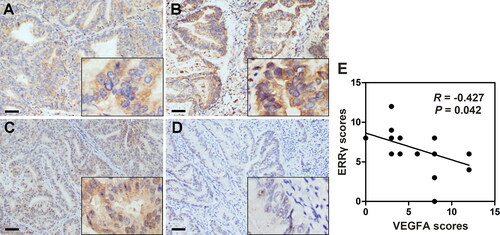
Figure 2. ERRγ reduces the expression and secretion of VEGFA in HEC-1A cells. A, Western blot analysis of the effect of ERRγ overexpression on protein expression of VEGFA in HEC-1A cells. HEC-1A cells were infected with a lentivirus harboring ERRγ (ERRγ) or a Mock lentivirus (Mock) for 48 h (multiplicity of infection = 2). Protein expression was measured relative to that in Mock-infected cells, with β-actin as the internal control. B, qRT-PCR analysis of the effect of ERRγ overexpression on mRNA expression of VEGFA in HEC-1A cells. C, ELISA analysis of the effect of ERRγ overexpression on secretion of VEGFA in HEC-1A cells. The concentration of VEGFA in the CMs collected from HEC-1A cells infected with a lentivirus harboring ERRγ (ERRγ) or a mock lentivirus (Mock). Protein band densities and RNA level were quantified, and β-actin serves as the internal control. Values represented the means (± SEM) of independent experiments (n = 3–4 per group). Graphs showed protein/mRNA expression relative to that in control cells (Mock). (*p < .05; **p < .01; ***p < .001). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; CM, conditioned medium.
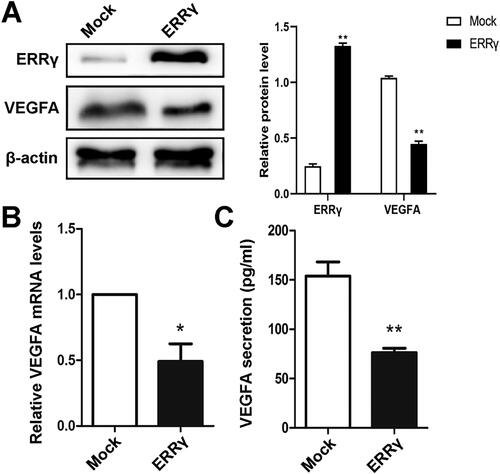
Figure 3. ERRγ inhibits angiogenesis in vitro and in tumor xenografts. A, Tube formation of HUVECs. 96-well dishes were coated with Matrigel, and HUVEC tube formation assays were performed 6 h after incubation with CMs collected from HEC-1A cells infected with a lentivirus harboring ERRγ or a mock lentivirus (Mock). Left, representative photographs were shown at 100 × magnification. Right, relative number of tubes per field were counted at 100 × magnification. B, Representative microphotographs of ERRγ, VEGFA and CD31 staining in nude mice tumor tissues (magnification: 400 ×, scale bar: 50 μm). C, for evaluation of the angiogenic index in nude mice tumor tissues, the CD31-positive blood vessels in the hotspot areas were counted at 400* magnification. Data represented the mean (± SEM) of 5 grafts in each condition. (*p < .05). D, the expression level of HIF in Mock group and ERRγ group was detected by immunohistochemistry. E–G, Representative tumor images (E), volume (F) and weight (G) of nude mice in Mock and ERRγ groups. (**p < .01). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; CM, conditioned medium; HUVEC, human umbilical vascular endothelial cells; HIF, hypoxia-inducible factor.
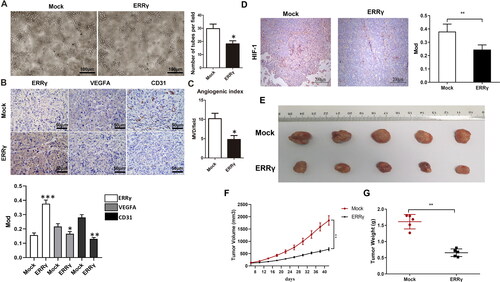
Figure 4. ERRγ inhibits hypoxia-induced proliferation and migration of HEC-1A cells. A, Identification of the interaction ERRγ with VEGFA by double luciferase reporter gene experiment. B, CCK8 method to detect cell viability. C, the wound healing test to cell migration. (**p < .01). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A.
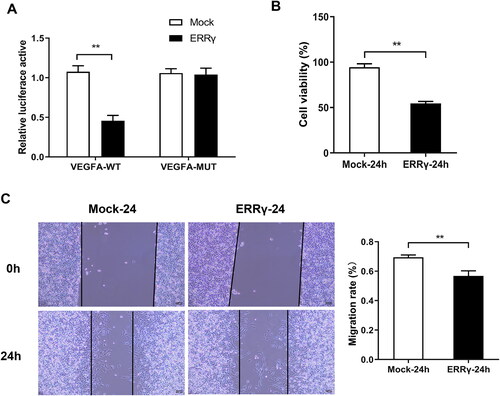
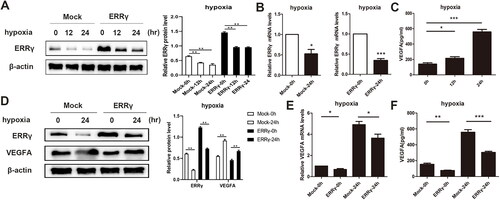
Figure 5. Reducing ERRγ expression by hypoxia induction and ERRγ upregulation can abolish hypoxia-induced expression of VEGFA. A, Western blot analysis of the effect of hypoxia on ERRγ protein expression in HEC-1A cells. ERRγ group and Mock group cells were incubated in hypoxic environment at the indicated oxygen levels for 12 h, 24 h, and those incubated in normoxic environment served as control. B, qRT-PCR analysis of the effect of hypoxia on ERRγ mRNA expression in HEC-1A cells. C, ELISA analysis of the effect of hypoxia on VEGFA secretion in HEC-1A cells. D, Western blot analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA protein expression. E, qRT-PCR analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA mRNA expression. F, ELISA analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA secretion. Protein band densities and RNA level were quantified, with β-actin as the internal control. Values represented the means (± SEM) of independent experiments (n = 3–4 per group). Graphs showed protein/mRNA expression relative to that in control cells. (*p < .05; **p < .01; ***p < .001).
Supplemental Material
Download MS Word (18 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
