Figures & data
Figure 1. E2 upregulated HOTAIR expression in Ishikawa cells. (a) The expression of HOTAIR in human endometrial cancer tissue (tumor) and the adjacent normal tissue (control) from endometrial cancer (EC) patients was determined by quantitative polymerase chain reaction (qPCR). ** P<0.01, n = 15 for each group. (b,c) The expression of HOTAIR in Ishikawa cells treated with various concentrations of estradiol (E2) (b), or treated with 10 nM E2 for 0, 1, 2, 4, 6, 8, or 10 h (c), was determined by qPCR. (d) The expression of HOTAIR in Ishikawa cells left untreated, treated with E2 alone, or treated with E2 plus estrogen inhibitor ICI 182780, was determined by qPCR. (e-f) The expression of HOTAIR in control Ishikawa cells and cells transfected with small interfering RNA against HOTAIR (siHOTAIR) (e) or infected with lentivirus expressing small hairpin RNA (shRNA) against HOTAIR (shHOTAIR) or a negative control shRNA (shNC) (f), was determined by qPCR. (g) Control Ishikawa cells and the cells infected with shNC or shHOTAIR-expressing lentivirus were treated with 10 nM E2 for 4 h. The expression of HOTAIR in the indicated 4 groups was determined by qPCR. *** P<0.001, n = 3 for each group.
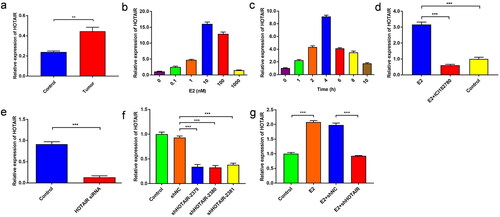
Figure 2. E2 promotes PRC2 expression and migration of Ishikawa cells via upregulated HOTAIR expression. (a–b) The protein expression of PRC2 (EZH2 and SUZ12) in Ishikawa cells treated with/without 10 nM estradiol (E2) for 4 h was determined by western blot assays. (c,d) The protein expression of PRC2 (EZH2 and SUZ12) in control Ishikawa cells and small interfering RNA against HOTAIR (siHOTAIR)-transfected Ishikawa cells after 10 nM E2 treatment for 4 h was determined by western blot assays. Representative band images are shown (a, c), and the relative protein levels were summarized (b,d). *** P<0.001, n = 3 for each group. (e–g) The migration capacity of Ishikawa cells was determined in the presence of E2 using the Transwell assay. The Ishikawa cells were treated without E2 (e) or with E2 (f). (h–j) The migration capacity of Ishikawa cells treated with E2 was determined with siHOTAIR-induced HOTAIR knockdown using the Transwell assay: without HOTAIR knockdown (h) and with HOTAIR-knockdown (i). Representative images of the Transwell assays are shown in e,f,h, and i, and the number of migrated cells per visual field was summarized (g,j). Magnification, 200 ×. *** P < 0.001, n = 3 for each group.
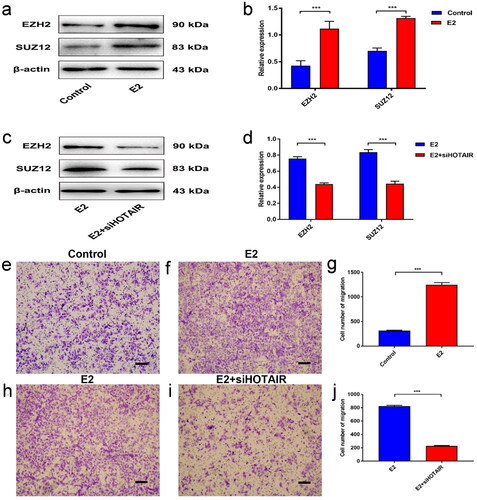
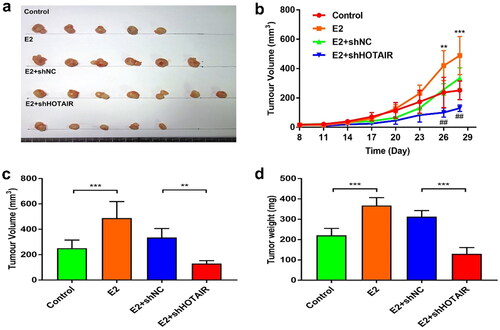
Figure 3. E2-enhanced in vivo proliferation of Ishikawa cells was dependent on HOTAIR expression. (a–d) Nude mice were inoculated with control Ishikawa cells or stable Ishikawa cells with lentivirus infection-mediated expression of small hairpin RNA (shRNA) against HOTAIR (shHOTAIR) or a negative control shRNA (shNC) on Day 0. Mice in the estradiol (E2) treatment groups were subcutaneously implanted with 17β-estradiol-releasing pellets (a) Images of tumors at day 28 from the mice indicated in the different groups. (b) The growth curve of xenograft tumors in the indicated groups (*, E2 vs. Control; #, E2 + shNC vs. E2 + shHOTAIR). (c,d) Xenograft tumor volume (c) and weight (d) at the endpoint (day 28) were measured. ** P<0.01, *** P<0.001, ## P<0.01, n = 5 for all groups in b, c, d.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
