Figures & data
Figure 1. Characterisation of platelets from Cttn KO and DKO mice. (A) Western blots of platelet lysates confirming phosphorylation of Cttn downstream of G-protein coupled and tyrosine kinase linked receptors. Cortactin was immuno-precipitated from stimulated mouse platelet lysates and western blotted with anti-phosphotyrosine (4G10) and anti-cortactin (4F11) antibodies. (B) Whole blood platelet counts and mean platelet volumes were measured from WT, Cttn and DKO mice. No significant difference was observed between the genotypes in either platelet number or volume. Data is mean ± SD (WT, n = 37; Cttn KO, n = 24; DKO, n = 18). (C) Platelet surface receptor levels were analysed by flow cytometry. No significant differences were observed between either genotype and WT controls. Data are % of WT control (Mean ± SEM; n = 3) and values were corrected for IgG background staining.
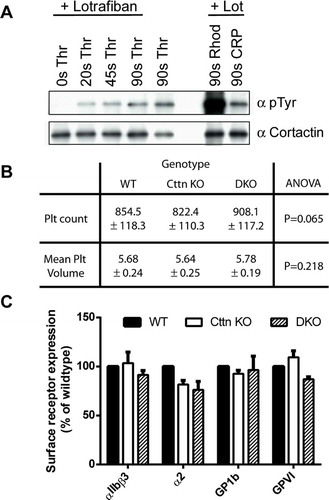
Figure 2. Loss of Cttn and HS1 does not affect platelet spreading or F-actin organisation. (A) The loss of Cttn only or Cttn and HS1 from platelets did not affect their ability to adhere and spread on fibrinogen (±0.1 U/ml thrombin) or collagen related peptide (CRP) coated coverslips. (B) Quantitation of the surface area of spread platelets from either Cttn KO or DKO platelets showed no significant differences in spreading. (C) Staining of spread platelets for F-actin with fluorescent phalloidin showed normal actin organisation with filopodia, actin nodules and platelet stress fibres being observed in both WT and DKO platelets. Scale bars in (A) = 10 µm and in (C) = 5 µm.
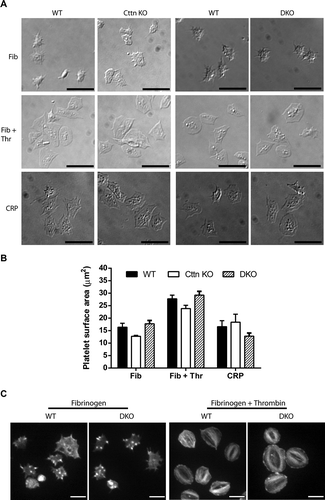
Figure 3. Loss of Cttn and HS1 does not affect platelet function. The loss of Cttn alone or both Cttn and HS1 had no effect on the aggregation (A & B) or α-granule secretion (C) of platelets to G-protein coupled or tyrosine kinase linked receptor agonists. Aggregation data are expressed as % final aggregation 6 mins after agonist addition. Alpha granule secretion data are % of WT values measured by flow cytometry 2 mins after addition of agonist. (D) The loss of Cttn and HS1 had no effect on pro-coagulant surface generation as phosphatidylserine exposure was not affected by loss of both cortactin and HS1. (E) Clot retraction in PRP following stimulation by thrombin was unaffected by loss of Cttn or Cttn and HS1. All data are presented as mean ± SEM, n = 3.
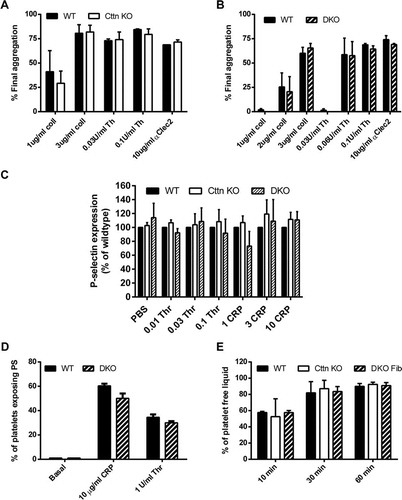
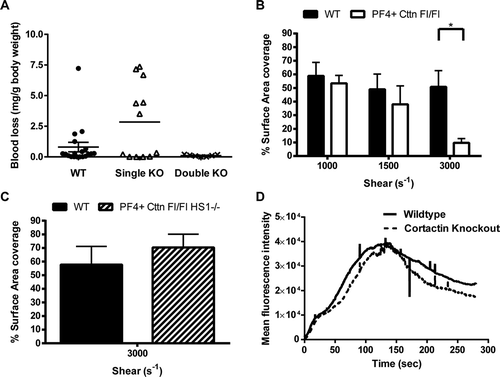
Figure 4. In vivo thrombosis assays and in vitro flow studies. (A) An increase in tail bleeding (mg blood loss/g body weight) was observed in Cttn KO mice following removal of the terminal 3 mm of the tail. However, this increase was not significant and was not observed in DKO mice. Symbols (● = WT, Δ = Cttn KO, × = DKO) represent individual data points, horizontal bars the mean and vertical bars the SEM (n = 18 for WT, 12 for Cttn KOs and 7 for DKOs). (B) In vitro flow assays performed over collagen showed no significant decrease in aggregate formation at shear rates of either 1000 s−1 or 1500 s−1 for either Cttn KO or DKO mice. (C & D) In vivo thrombosis, as determined by the cremaster laser injury model, showed no effect of loss of Cttn on either thrombus size (C) or time to peak intensity (D).