Figures & data
Figure 1. Methods to investigate platelet-leukocyte aggregate (PLA) formation. (A) Flow cytometry represents a high-throughput approach to quantify circulating PLAs in human and animal blood and to investigate expression of involved molecules. Due to its fast protocol and independence of highly specific machines, standard flow cytometry is readily usable in clinical settings. Panels show gating strategy to determine platelet hetero-aggregates with distinct leukocyte subtypes in stimulated murine blood. CD45 (pan-leukocyte marker), Ly6G (neutrophil marker), CD11b (marker to exclude cells other than lymphocytes) and CD41 (pan-platelet marker). (B) Tissue sectioning adds information about location of PLAs within human or animal tissues in conjunction with quantitative and qualitative evaluation of PLAs. However, preparation and evaluation of samples is more time-consuming and does not allow for high-throughput analysis, making tissue sectioning less attractive for clinical laboratories. Images show PLAs in inflamed murine lung histology sections (neutrophils labelled with anti-CD45 and platelets with anti-CD41). C) Concomitant live analysis of multiple cell types in animals in vivo or of human or animal blood cells in microfluidic devices provides information about dynamic changes of platelet-leukocyte interactions and their effect on cellular effector functions within physiologic microenvironments or under semi-physiological conditions under flow. Live cell imaging to investigate PLAs requires state-of-the-art microscopy facilities and areas of interest that are accessible for microscopy, thus making it well suited for basic research but not for clinical applications. Panel shows multiphoton image sequences of a platelet (labelled with anti-CD49b) interacting with a GFP+ neutrophil within glomerular capillaries of a LysM-GFP mouse over time (endothelial cells labelled with anti-CD31). PLA: platelet-leukocyte aggregate, SSC-A: side scatter––area, DAPI: 4′,6-Diamidin-2-phenylindol.
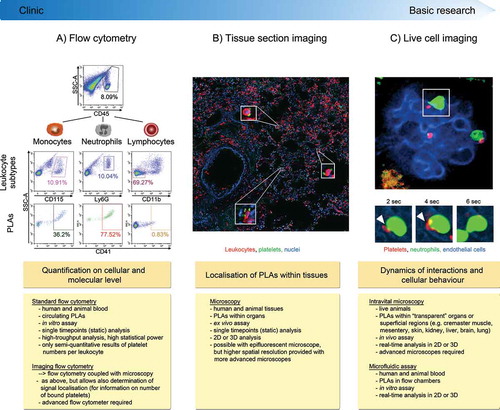
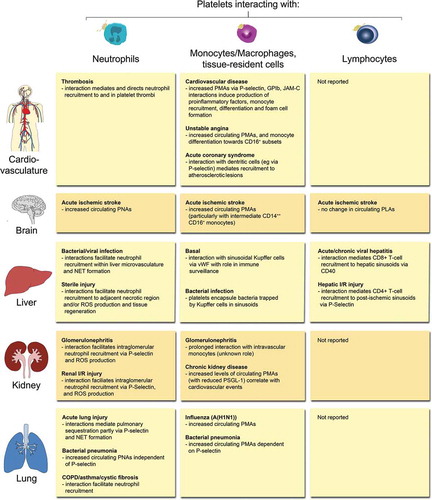
Figure 2. Overview of PLA formation and their effects in (thrombo-) inflammatory pathologies of selected organs. Physical interactions of platelets with neutrophils, monocytes/macrophages, tissue-resident leukocytes or lymphocytes have been reported in a number of (thrombo-)inflammatory diseases of the circulatory system, brain, liver, kidney, and lung. Effects of platelet-leukocyte binding on cellular and/or molecular responses are summarised for individual leukocyte subtypes. COPD: chronic obstructive pulmonary disease, I/R: ischemia/reperfusion, JAM-C: Junctional adhesion molecule-C, NET: neutrophil extracellular trap, PMA: platelet-monocyte aggregate, PNA: platelet-neutrophil aggregate, PSGL-1: P-selectin glycoprotein ligand 1, ROS: reactive oxygen species, vWF: von Willebrand factor.