Figures & data
Figure 1. Coronins present in human and mouse platelets and subcellular distribution of human Coro1. (A) Western blot of human and mouse platelet lysates. Twenty micrograms of protein were resolved by 10% SDS-PAGE, blotted onto PVDF membrane and probed with antibodies for the indicated proteins. The mouse Coro2 blot corresponds to a higher exposure than the human one. The Coro7 blot was enhanced to make the human protein apparent (see Supplemental for details). GAPDH was used as a loading control. (B) Subcellular fractionation. Human platelets were lysed by freeze-thaw in liquid nitrogen and spun at 100,000 × g for 1 h to separate membrane (M) and cytosolic (C) fractions. The fractions were normalized by volume and resolved by 12% SDS-PAGE, blotted onto PVDF membrane and probed with antibodies for the indicated proteins. CD36 was used as a membrane marker and Syk as a cytosolic marker in resting platelets. Latrunculin B (LatB; 20 µM, 20 min) was used to depolymerize F-actin prior to lysis. Coro1 and actin distribution were quantified by densitometry and expressed as a percentage relative to the respective totals (M + C). (C) Association of Coro1 to actin in the detergent-insoluble pellet. Human platelets (8 × 108/ml) were lysed in the presence of 1% Triton X-100 and lysates spun at low speed (15,600 × g) for 20 min and high speed (100,000 × g) for 1 h. Supernatant (S) and pellet (P) fractions were normalized by volume and resolved by 12% SDS-PAGE, blotted onto PVDF membrane and probed with antibodies for the indicated proteins. LatB (20 µM, 20 min) was used to depolymerize F-actin prior to lysis. Coro1 and actin distribution in pellet and supernatant were quantified by densitometry and expressed as a percentage of the respective total (P + S). Data of B and C represent mean ± SD of three independent experiments. **P< .01, ***P < .001 vs LatB-treated, Student’s t-test.
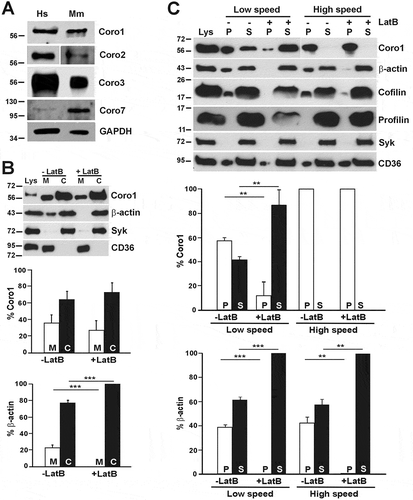
Figure 2. Subcellular localization of Coro1. Human platelets were fixed in suspension with paraformaldehyde and spun on poly-L-lysine coated coverslips (A) or were allowed to spread on 100 µg/ml fibrinogen (B, D) or collagen (C) coated coverslips and fixed with paraformaldehyde. For A, B, C and D cells were immunostained with an anti-Coro1 antibody followed by an Alexa568-coupled secondary antibody (red) and counterstained with FITC-phalloidin for filamentous actin (green). For E platelets were treated with 100 nM PGI2 at 37°C 5 min prior to fixation in order to increase the proportion of cells displaying actin nodules. Platelets were then immunostained with anti-Coro1 and anti-CAP1 antibodies followed by Alexa568 and Alexa488-coupled secondary antibodies, respectively (red and green), and counterstained with Alexa680-phalloidin for filamentous actin (blue). Actin color has been changed to red in the double staining panel with CAP1 for better visualization. Images were acquired with a fluorescence microscope equipped with a structured illumination attachment and deconvolved. Magnified regions are indicated with a square. Arrows point at regions of interest: cell cortex (A, B), actin filaments (C), actin nodules (D, E). Arrowheads in B point at Coro1 along stress fibers. Scale bars 5 µm. The scale bar on A applies to B, C, and D.
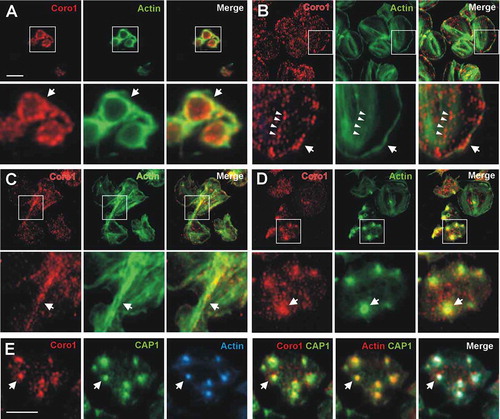
Figure 3. Subcellular localization of Coro1 upon disruption of the actin and tubulin cytoskeletons. Human platelets were incubated with 3 µM LatB or 10 µM nocodazole for 30 min, fixed in suspension with paraformaldehyde and spun on poly-L-lysine coated coverslips. Cells were immunostained with anti-Coro1 and anti-tubulin antibodies followed by Alexa568 and Alexa488-coupled secondary antibodies, respectively (red and green), and counterstained with Alexa680-phalloidin for filamentous actin (blue). Images were acquired with a fluorescence microscope equipped with a structured illumination attachment and deconvolved. Arrows indicate the magnified cell shown in the second row of each treatment. Scale bar 5 µm.
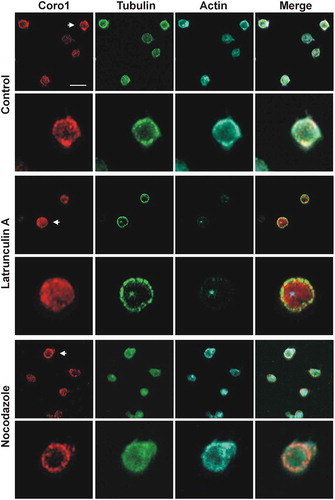
Figure 4. Subcellular localization of Coro2 and 3. Human platelets were fixed in suspension with paraformaldehyde and spun on poly-L-lysine coated coverslips (A, D) or were allowed to spread on 100 µg/ml fibrinogen coated coverslips (B, E, C, F) and fixed with paraformaldehyde. Cells were immunostained with an anti-Coro2 or Coro3 antibody followed by an Alexa568-coupled secondary antibody (red) and counterstained with FITC-phalloidin for filamentous actin (green). Images were acquired with a fluorescence microscope equipped with a structured illumination attachment and deconvolved. Magnified regions are indicated with a square. Arrows point at regions of interest: cell cortex (A, B, D, E), actin nodules (C, F). Scale bar 5 µm.
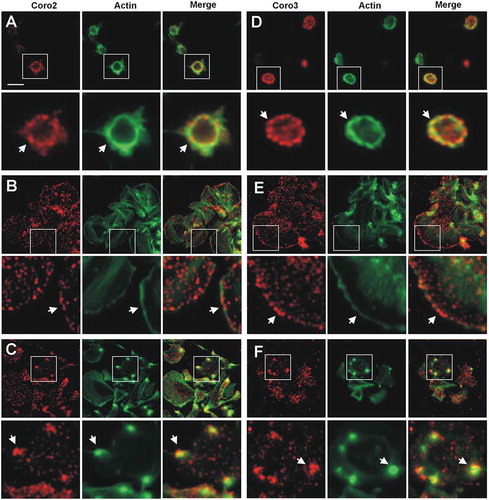
Figure 5. Coronins exist in complexes with each other and with Gαs and the Arp2/3 complex. (A) Human platelet lysates were subject to immunoprecipitation with Coro1 or Coro2-specific antibodies. The same species of the total immunoglobulin G (IgG) was used as a control. Protein complexes were examined by Western blot for the presence of the indicated proteins. (B) Colocalization of Coro1 with Gαs and ARPC2. For Gαs platelets were fixed in suspension with paraformaldehyde and spun on poly-L-lysine coated coverslips. For ARPC2 platelets were allowed to spread on 100 µg/ml fibrinogen coated coverslips and fixed with paraformaldehyde. Cells were immunostained with anti-Coro1 and anti-ARPC2 or anti-Gαs antibody followed by Alexa568 or Alexa488-coupled secondary antibodies, respectively (red and green). Images were acquired with a fluorescence microscope equipped with a structured illumination attachment and deconvolved. Arrows point at regions of apparent colocalization. Scale bar 5 µm. (C) Coronins colocalize with each other. Platelets were allowed to spread on 100 µg/ml fibrinogen coated coverslips, fixed with paraformaldehyde, immunostained with the indicated coronin antibodies followed by Alexa568 or Alexa488-coupled secondary antibodies, respectively (red and green), and counterstained with Alexa680-phalloidin for filamentous actin (blue). Images were acquired as in (B). Arrows point at regions of interest: cell cortex (upper panels), actin nodules (lower panels). Scale bar 5 µm.
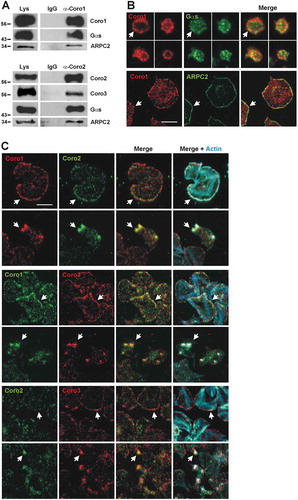
Figure 6. Dynamics of Coro1 along with F-actin in the Triton X-100 insoluble pellet of platelets stimulated with thrombin or collagen. Washed human platelets were stimulated with thrombin (0.1 U/ml) or collagen (50 µg/ml) at the indicated time points and lysed immediately in 1% Triton X-100 lysis buffer. Triton insoluble pellets were prepared by low-speed centrifugation (15,600 × g for 15 at 4°C), run on SDS-PAGE and subjected to Western blot analysis with anti-Coro1, Coro3 and β-actin antibodies. Densitometry values are expressed as means ± SEM of 3–7 experiments *P < .05, **P< .01, ***P < .001 relative to basal, student’s t-test.
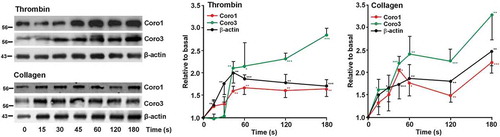
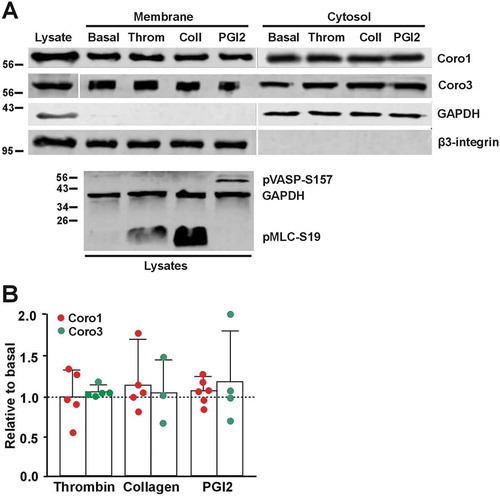
Figure 7. Coronins do not translocate upon platelet stimulation. (A) Washed human platelets (8 × 108/ml) were treated with 1 mM EGTA, 10 nM indomethacin and 2 U/ml apyrase for 20 min at 37°C to prevent aggregation. They were then treated with 0.1 U/ml thrombin, 50 µg/ml collagen or 100 nM PGI2 for 1 min at 37°C prior to lysis and subcellular fractionation. Fractions were normalized by volume and resolved on 12% SDS-PAGE, blotted onto PVDF membrane and probed with antibodies for Coro1 and Coro3. Integrin β3 was used as a membrane marker and GAPDH as a cytosolic marker. The phosphoproteins pMLC-S19 and pVASP-S157 were used as markers of the effects of thrombin/collagen and PGI2, respectively, and GAPDH as a loading control. (B) Membrane-associated Coro1 and Coro3 upon stimulation were quantified by densitometry, normalized to integrin β3 and expressed relative to the respective coronin in the basal membrane fraction. Data represent the mean ± SEM of 3–6 independent experiments. No statistically significant differences were found relative to basal for any coronin using Mann-Whitney U and Kruskal-Wallis tests.