Figures & data
Figure 1. A) Schematic overview of the experimental set-up to monitor phagocytosis of opsonized platelets: CD14+ monocytes were cultured for 9 days with GM-CSF to differentiate into monocyte-derived macrophages (MQ M1). Hereafter, the macrophages were pre-incubated with and without FcγR-blockers. Freshly isolated platelets from HLA-A2+ donors were labeled with PKH26 and pre-incubated with unmodified and glycoengineered anti-HLA monoclonal antibodies in the presence of complement sufficient or heat-inactivated (HI) serum, for antibody and complement opsonization. The macrophages and platelets were washed and co-incubated for 30 minutes at 37°C and analyzed by flow cytometry and Imagestream B) Expression levels of complement receptor 3 (Cd11b/cd18) and FcγR’s (FcγRI, FcγRII, FcγRIII) on the surface of the monocyte-derived macrophages as analyzed by flow cytometry. C-E) Internalization of platelets was determined employing C) conventional and D-E) imaging flow cytometry. Anti-CD42a-BV421 staining, in combination with PKH26, was used to identify platelets attached to the exterior of the cell. Representative images are shown for indicated quadrants obtained by imaging flow cytometry.
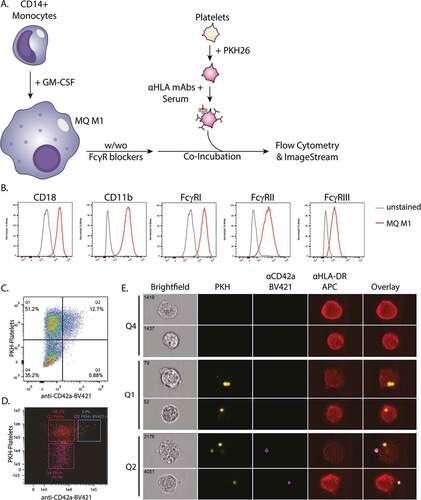
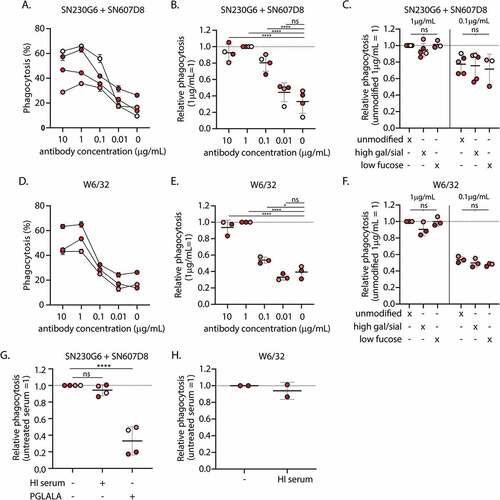
Figure 2. Phagocytosis of complement and antibody opsonized platelets by monocyte-derived macrophages A) Absolute and B) relative levels of phagocytosis of platelets incubated with various concentrations of unmodified hIgg1 anti-HLA monoclonal antibodies (mAb) SN230G6+SN607D8 in the presence of complement sufficient serum C) Differences in phagocytosis between platelets pre-incubated with unmodified and glycoengineered hIgg1 SN230G6+SN607D8 mAbs in the presence of complement sufficient serum. D) Absolute and E) relative levels of phagocytosis of platelets pre-incubated with various concentrations of unmodified pan-anti-HLA Class I hIgg1 mAb W6/32 in the presence of complement sufficient serum F) Differences in phagocytosis between platelets pre-incubated with unmodified and glycoengineered hIgg1 W6/32 mAb in the presence of complement sufficient serum. G-H) Relative phagocytosis levels of platelets pre-incubated with 1 µg/ml unmodified or PG LA LA Fc mutant anti-HLA mAbs (SN230G6+SN607D8 or W6/32) in the presence of complement sufficient or heat-inactivated (HI) Serum A-H) the level of phagocytosis (%) was defined as the percentage of the PKH26+ macrophage fraction (Q1+Q2). The data represents the mean and SD of 2 technical replicates of 2–5 different monocyte donors used in 2–4 independent experiments, for each independent experiment also a different platelet donor was used. The color of the data points indicates the different monocyte donors. For normalization, the level of phagocytosis of platelets incubated with 1 µg/ml unmodified anti-HLA mAbs was set at 1, as indicated. An ordinary one-way ANOVA with Dunnet’s multicomparison test was performed for the statistical analysis. *p ≤ .05, ****p ≤ .0001 and ns = non-significant .