Figures & data
Figure 1. Schematic representation of GALE cDNA and protein. (a) Schematic representations of GALE cDNA and UDP-galactose 4-epimerase (GALE protein). GALE encodes a 348 amino acids (aa) protein containing multiple sites for NAD+ binding (blue) (aa from Gly12 to Ile14 (Gly12-Ile14), Asp33-Asn37, Asp66-Ile67, Phe88, Lys92, Lys161, and Tyr185), substrate UDP-glucose binding (green) (aa Ser132-Thr134, Tyr185-Asn187, Asn206-Leu208, Asn224-Phe226, Arg239, and Arg300-Asp304), and a proton acceptor site (yellow) (aa Tyr157). (b) Structural 3D representation of GALE (Rcsb:1ek6). The protein is colored gray, with NAD+ binding sites in light blue, UDP-glucose-binding sites in green, and the proton acceptor site in yellow. NADH molecule and UDP-glucose are represented in dark blue and light green, respectively. Image was created using ChimeraX 1.3 software.
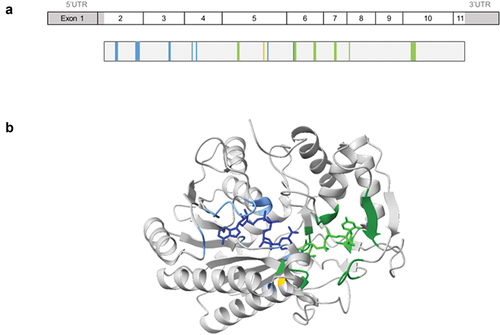
Figure 2. Role of the UDP-galactose 4-epimerase in O-linked and N-linked glycosylation. GALE protein allows the interconversion of UDP-glucose and UDP-galactose, and that of UDP-N-acetyl-galactosamine and UDP-N-acetyl-glucosamine. These four molecules are essential in the glycosylation process, by serving as substrates for other enzymes incorporating the carbohydrates of interest and releasing UDP. Branches of carbohydrates are essential in both N-glycosylation and O-glycosylation processes.
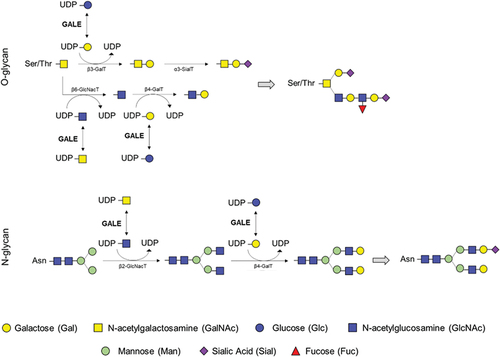
Figure 3. Schematic illustration of the clinical phenotype in patients with peripheral, intermediate, and generalized galactosemia III. in peripheral galactosemia, GALE dysfunction is restricted to the circulating blood cells, especially red blood cells, and the common clinical manifestation is galactosemia. In intermediate/generalized galactosemia, GALE dysfunction can be detected in multiple tissues, and therefore, patients present with syndromic manifestations, that may include neurological disorders, learning difficulties, delay growth, sensorineural hearing loss, early-onset cataracts, cardiac failure, hepatomegaly and hyperbilirubinemia, and pancytopenia associated with increased bleeding tendency. Image resource: Servier Medical Art (https://smart.servier.com/).
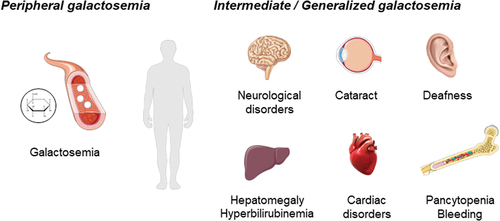
Figure 4. Variants associated to peripheral, intermediate, and generalized forms of the disease. Reported variants among the GALE protein. Variants marked in red indicate variants causing generalized forms of the disease associated with hematological manifestations. NAD+ binding is represented in blue, substrate UDP-glucose binding in green, and the proton acceptor site in yellow.
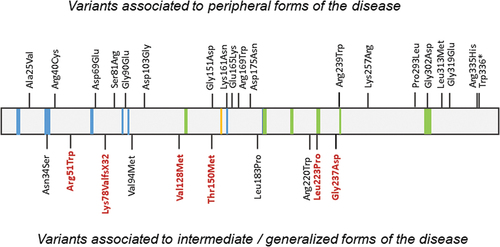
Table I. Molecular, clinical, and laboratory presentation of patients with GALE variants and generalized galactosemia.
