Figures & data
Figure 1. Activation of blood platelets by F11 antibodies binding to F11R/JAM-A. F11 bind to F11R/JAM-A and Fc fragment of the antibodies is recognized by FcγRII receptor which triggers platelet activation.
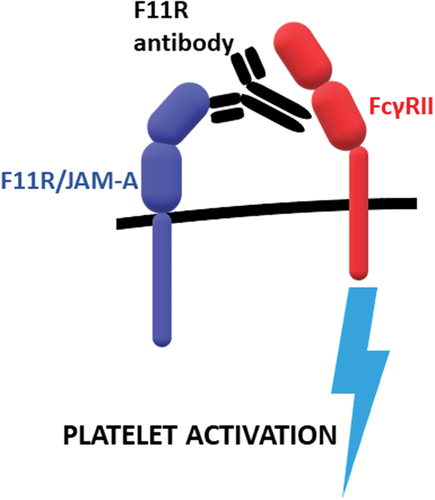
Figure 2. Structure and molecular interactions of the extracellular component F11R/JAM-A a) structure of F11R/JAM-A, b) cis-homophilic dimerization – two molecules of F11R/JAM-A located in the same cell interact with each other via D1 domain, c) trans-homophilic interactions – two, or more F11R/JAM-A dimers located on adjacent cells interact with each other via D1 domain, the site of interaction is different from the one responsible for cis-homophilic dimerization, d) heterophilic interaction with lymphocyte function-associated antigen (LFA-1) - LFA-1 is the only identified to date heterophilic ligand for F11R/JAM-A, the interaction occurs via D2 domain.
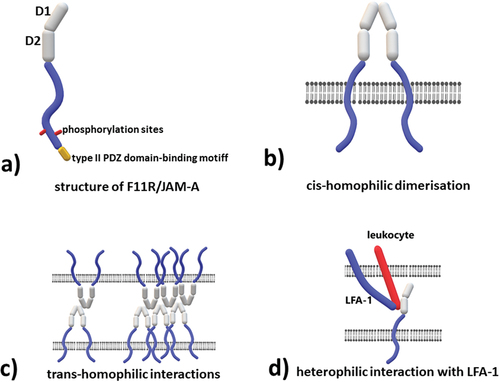
Figure 3. Interaction of F11R/JAM-A with αIIbβ3. Monomeric F11R/JAM-A interacts with αIIbβ3 via tetraspanin CD9.
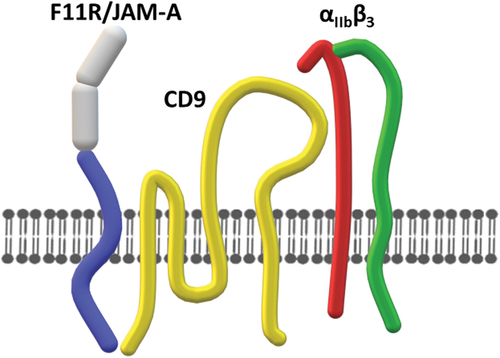
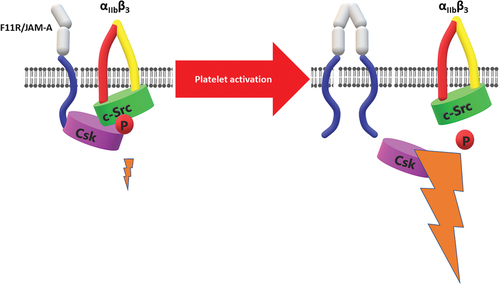
Figure 4. F11R/JAM-A regulates outside-in signaling of αIIbβ3. In resting platelets Csk kinase is associated with F11R/JAM-A and remains in proximity to αIIbβ3-associated c-Src kinase. Phosphorylation of c-Src mediated by Csk limits outside-in signaling of αIIbβ3. Dissociation of F11R/JAM-A from the proximity of αIIbβ3 upon platelet activation, or F11R/JAM-A deficiency results in cessation of Csk inhibitory effect on c-Src. This in turn enhances outside-in signaling from the integrin.