Figures & data
Figure 1. The immunologic function of platelets. (a) Activated platelets secrete a large number of biological response regulators (BMRs). If patients with previous inflammatory pathological conditions or external factors attracting leukocytes to lung vessels, the BMRs contained in the transfused platelet concentrations will activate neutrophils to produce ROS, which damages the pulmonary endothelium and cause transfusion-related acute lung injury. Moreover, the growth factors are associated with angiogenesis; (b) Platelets express immune receptors, and Toll-like receptors (TLR) are involved in immune regulation. During heparin treatment, if patients with anti-PF4/polyanion antibodies (These antibodies probably result from bacterial infections or heparin treatment), their Fab parts will bind to PF4/heparin complexes, and then FcγRIIA on the platelet surface can bind to the Fc parts of anti-PF4/polyanion antibodies leading to HIT. TLR4 can bind to damage-associated molecular patterns (DAMPs, e.g. HMGB1) to trigger platelet activation and the release of BMRs. (c) Platelets can interact directly or indirectly with bacteria to achieve a bactericidal effect. Lipopolysaccharides on the surface of bacteria activate platelets and promote the production of PF4, which can bind to the negative charge of the bacterial surface, exposing a new epitope. This epitope is bound by anti-PF4/polyanion antibodies, promoting phagocytosis of the bacteria by granulocytes. Platelets can also internalize IgG-encapsulated bacteria to achieve bactericidal effects.
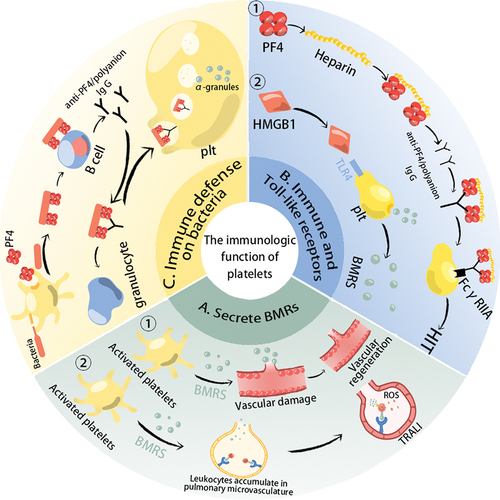
Figure 2. The mechanism of PEVs produced by platelet during storage. With the intracellular Ca2+ level was gradually increased during platelet storage, the membrane phospholipid molecules have been asymmetrically changed, which promotes the generation of platelet microvesicles (PMVs). The platelet exosomes (P-exos) generation begins with the membrane bulging into the lumen of endsome, which is converted into a multi-vesicular body (MVB), then the MVBs fuse with the plasma membrane to secrete exosomes. Both PMVs and P-exos belong to PEVs. The biomarkers and contents of platelet-derived extracellular vesicles (PEVs) usually contain CD62P, CD42a, CD9, CD63, CD41b, PF4, and RNA.
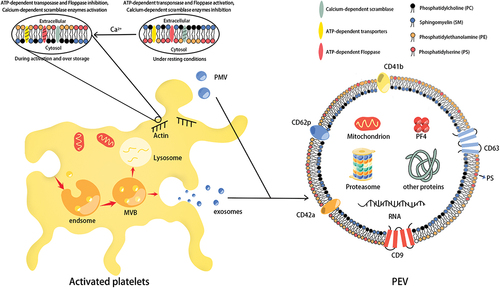
Table I. Commonly used methods and differences in isolation of PEVs.
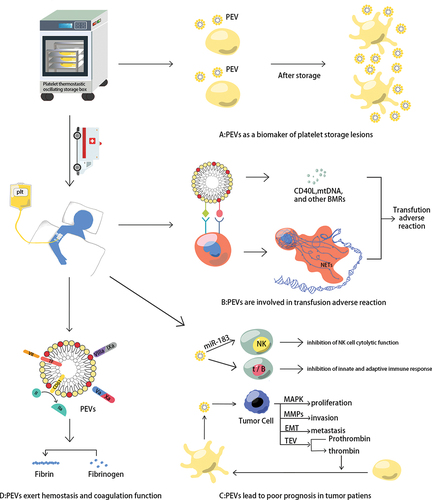
Figure 3. PEVs contributions in platelet transfusion therapy. (a) PEVs as a biomarker of platelet storage lesions. With the prolongation of storage time, the number of platelets does not change much, but the amount of PEVs in PCs is doubled, and the level of CD62p (the gold standard for the evaluation of PLT function) expression on platelet surface is negatively correlated with PEVs level; (b) PEVs can facilitate their adhesion to leukocytes, then several BRMs produced by PEVs and neutrophil extracellular traps (NETs) formed by activated neutrophils are associated with the inflammatory response; (c) PEVs can inhibit the innate and adaptive immune response in the tumor microenvironment (TME). What’s more, PEVs could promote the proliferation, invasion, and metastasis of tumor cells; (d) PEVs can promote hemostasis and coagulation. Factor VII on the PEVs surface combines with TF to activate the coagulation cascade; PS on the PEVs surface provides a catalytic surface for coagulation and promotes prothrombin and tenase complex formation; and CD61 on the PEVs surface promotes fast thrombin generation and assists in fibrin clotting.