Figures & data
Figure 1. Contribution of platelets in cancer. Platelets get activated by soluble factors and chemokines present in the tumor microenvironment and play a vital role in cancer progression. Platelets play crucial role in supporting cancer growth by inducing stable angiogenesis and neovascularization. They also promote epithelial to mesenchymal transition of tumor cells thereby facilitating metastasis. High platelet counts contribute to chemoresistance by countering the inhibitory effect of chemotherapeutics thereby facilitating tumor cell proliferation and disease spread. Created with BioRender.com.
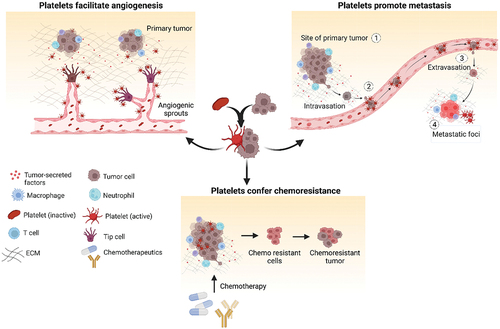
Figure 2. Organ-on-chip model of ovarian cancer vessel–platelet interaction, vascular degradation, metastasis and RNA-sequencing data analysis. (a) Schematic showing OvCa-chip components and assembly in detail. (b) Microscopic images showing human ovarian cancer cells, A2780 cultured in the upper microchannel (top) of OvCa-chip (center) and endothelial vessel formed in lower channel (bottom). Bars represent 100 µm. (c) Fluorescence micrographs displaying cross sectional view of the OvCa-chip with A2780 (red, anti-ZO1) cocultured with primary endothelial cells (green, VE-cadherin) to form a blood-perfusing vessel. The tumor-vascular tissue interface distance of cross-sectional 3D view is 10 µm. Bars represent 100 µm. (d) Immunohistochemical images of tumor biopsies obtained from healthy individual and patient with ovarian cancer showing platelets extravasation (CD42b+, brown and marked by arrows) into the tumor stroma in patients with and without statin treatment. Scale bar = 50 µm. Insets: mag = 40×. (e) Quantification of platelets observed with histology. Error bars are means ± SEM; n = 5. (f) Computer aided design of OTME-chip showing two PDMS compartments separated by thin porous membrane reproducing the microarchitecture of tumor-vascular interface. (g) OTME-Chip schematic depicting the influence of extravasated platelets in tumor invasion dynamics. (h) Cross-sectional 3D confocal view of OTME-Chip representing cancer cells (yellow), endothelial cells (red), and platelets (cyan) at 0 hours (left) and 72 hours (right) after platelet extravasation. Scale bars, 100 μm. (i) Volcano plots exhibiting differentially expressed genes in OTME-Chip, Cx-OTME-Chip, CxRx-OTME-Chip, and KO-OTME-Chip respectively relative to control (black dots). Red dots mark the genes regulating EMT and metastasis. (j) KEGG pathway clustering shows differential presence of EMT regulatory pathways for all study groups relative to control-chip (P < .05). Adapted with permissionCitation70 Copyright 2020, ASH publications and copyright 2021, Science publishing.
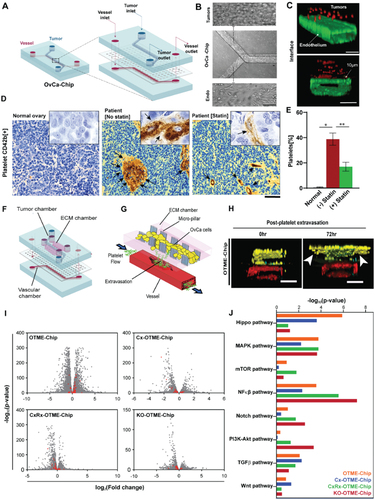
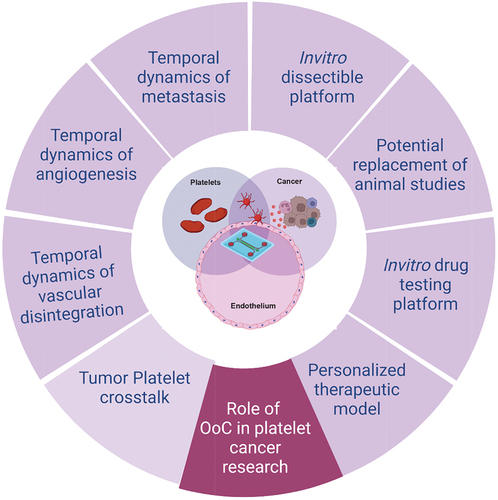
Figure 3. Importance of organ-on-chip (OoC) technologies in platelet cancer research. OoCs are the next generation modeling system facilitating in vitro disease modeling by closely mimicking the in vivo conditions in humans. This tool provides a dissectible platform by introducing flow mechanism and interaction of multiple cell types in real time by forming microchambers. OoC technology can be widely employed to better understand the role of platelets in vascular stress, angiogenesis and metastasis in cancer as well as finding new therapeutic targets and personalized therapeutic strategy for better disease management. Created with BioRender.com.