Figures & data
Figure 1. Syk gene structure and protein isoforms. The Syk gene consisting of 13 coding exons (2–14) and two 5’ untranslated exons 1a and 1b is presented schematically. The full-length protein SykL (629 amino acid-long in mice) and two relevant alternatively spliced isoforms – the previously characterized short isoform SykS (606 amino acid-long) lacking the sequence encoding by exon 7 and the hypothetical isoform corresponding to the mouse transcript designated Syk-202 (583 amino acid-long) lacking sequences encoded by exons 7 and 14 – are shown. The deletion/truncation locations, their borders and the exons corresponding to them as well as the major protein domains of Syk are indicated. The protein-length scale is shown at the bottom of the figure.
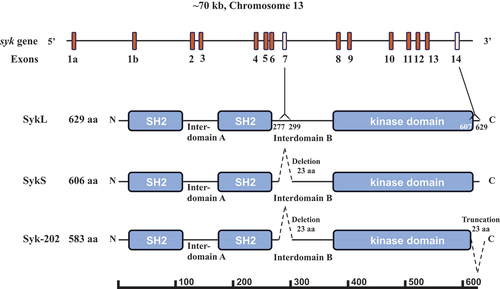
Figure 2. Molecular masses of Syk isoforms in various tissues. (A) lysates of human and mouse platelets were resolved using SDS-PAGE and probed with SYK-01, a monoclonal antibody raised against the 5-360aa region of Syk. (B) lysates of various mouse cells and tissues as indicated have been analyzed as described in (A). The molecular masses of Syk isoforms determined using protein standards are indicated in (A) and (B). The data are representative of at least three independent experiments from three different human donors and mice.
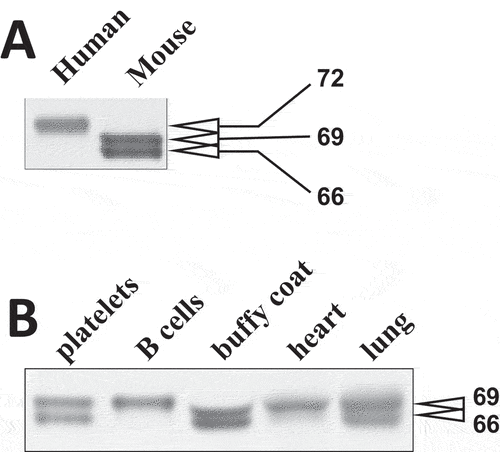
Figure 3. Exon 7-encoded amino acid sequence is present in the top mouse Syk isoform. (A) the Syk sequence encoded by exon 7, the numbers of amino acid residues corresponding to its borders, and SER291, a stimulation-induced phosphorylation site located within this sequence, are presented on a diagram. (B) WT mouse platelets were stimulated with CRP at different concentrations or left non-stimulated as indicated. Platelet lysates were resolved using SDS-PAGE and analyzed as described in the legend of except that pSER291 was also probed with a specific anti-pSER291 antibody. The positions of protein standards are indicated schematically with bars and presented on the immunoblots (intense red bands). Their molecular masses in kDa are indicated. The results are presented as individual panels for anti-pSER291 and anti-Syk (B) or a combination panel (C). The data are representative of at least three independent experiments.
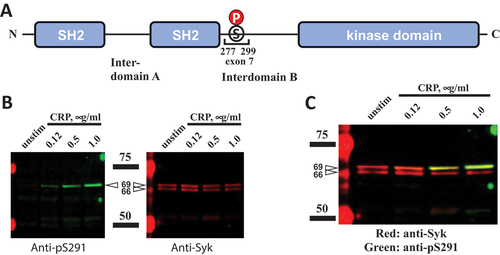
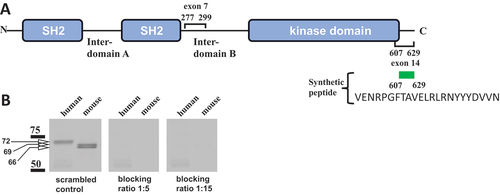
Figure 4. Exon 14-encoded C-terminal amino acid sequence is present in all Syk isoforms examined. (A) the Syk sequences encoded by exons 7 and 14 and their borders are indicated on a diagram. The synthetic peptide corresponding to the 613-629aa Syk sequence, which was used for blocking, is shown. (B) human and mouse platelet lysates were analyzed as described in the legend of fig. 2 with two modifications: SDS-PAGE-separated proteins were probed with an antibody raised to the C-terminal fragment of Syk (CST-2712), which was preincubated with the Syk 607–629 peptide VENRPGFTAVELRLRNYYYDVVN (blocking) or a scrambled peptide (control) for 30 min at room temperature. Blocking by the 607–629 peptide was examined at two different concentrations as indicated. The positions of protein standards and their molecular masses in kDa are indicated. The data are representative of at least three independent experiments.