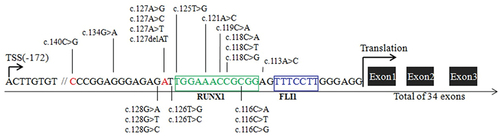
Figures & data
Table I. Patients’ clinical characteristics.
Table II. Patients’ laboratory characteristics.
Figure 1. Representative chromatograms from direct sequencing of the ANKRD26 gene. The red arrow indicates the mutation position. (a) represents the mutation c.-140C>G of ANKRD26 in 5’UTR in patient 1. (b) represents the mutation c.-127A>T of ANKRD26 in 5’UTR in patients 2.
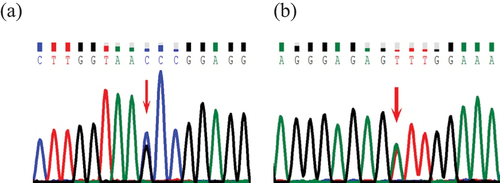
Figure 2. (a) Therapeutic effect of immunosuppressor in patient 1. (b) Therapeutic effect of immunosuppressor in patient 2.
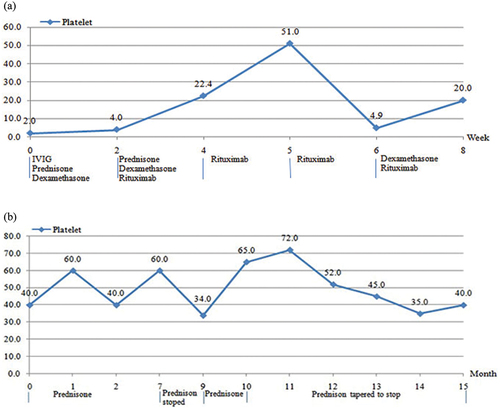
Table III. Therapeutic effect of immunosuppressor.
Table IV. Therapeutic effect of eltrombopag.