Figures & data
Figure 1. Cellular stiffness is decreased in human platelets and wild-type (C5BI6/J) murine platelets when treated with 8-Br-cGMP, riociguat or cinaciguat. (a,d,g) topography and cellular stiffness images of washed human, wild-type murine, and platelet-specific NO-GC KO murine platelets. (b,e,h) cellular stiffness quantification. (c,f,i) Western blot analysis of VASP phosphorylation (p-VASP) and NO-GC. GAPDH was used as loading control. DMSO (1:1000), 8-Br-cGMP (1 mM), riociguat (10 μM), cinaciguat (10 μM). The significance level of P-values is indicated by asterisks (* p < .05; ** p < .01; *** p < .001; ns, not significant; Tukey’s test). Scale bars: 5 μm. SICM experiments were performed with 4–24 platelets per donor from n = 2 (human), n = 2 (wild-type mice), n = 2 (NO-GC KO mice) donors. Western blots were performed for n = 3 (human), n = 3 (wild-type mice), n = 4 (NO-GC KO mice) donors.
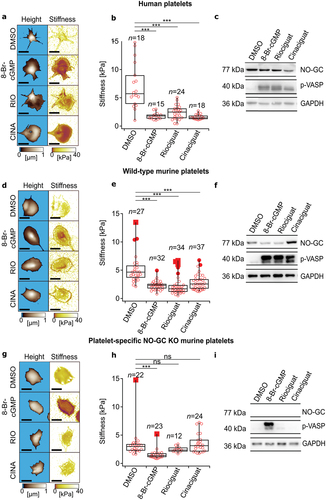
Figure 2. Platelet shape is altered in human platelets and wild-type (C57Bl6/J) murine platelets when treated with 8-Br-cGMP, riociguat, or cinaciguat. (a,d,g) phase contrast and binary prediction images obtained via deep learning morphometry in washed human, wild-type murine, and megakaryocyte/platelet-specific NO-GC KO murine platelets. (b,e,h). platelet circularity. (c,f,i) platelet area. DMSO (1:1000), 8-Br-cGMP (1 mM), riociguat (10 μM), cinaciguat (10 μM). The significance level of P-values is indicated by asterisks (*p < .05; **p < .01; ***p < .001; ns, not significant; Dunn’s test). Scale bars: 5 μm. Experiments were performed with ≈ 15 platelets per donor from n=4 (human), n=4 (wild-type mice), and n=4 (NO-GC KO mice) donors.
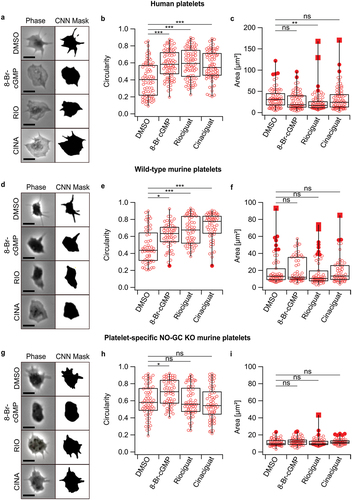
Figure 3. Riociguat increases platelet circularity and decreases platelet aggregation in HIV-negative volunteers, but not in HIV-positive patients taking ABC-containing regimens. (a, b) platelet circularity. (c, d) maximum of ADP-induced aggregation in response to indicated drug treatments. ADP (3 µM), DMSO (1:1000), CBV-TP (50 µM), riociguat (10 µM), ODQ (20 µM). Platelet numbers for circularity ≈ 15 per donor from n=2 donors. Donors for aggregation experiments: n=3. The significance level of p-values is indicated by asterisks (* p < .05; ** p < .01; *** p < .001; ns, not significant; Tukey’s test).
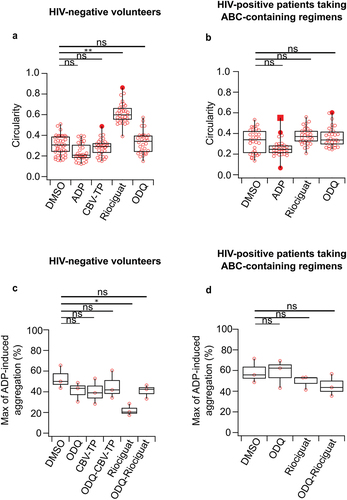
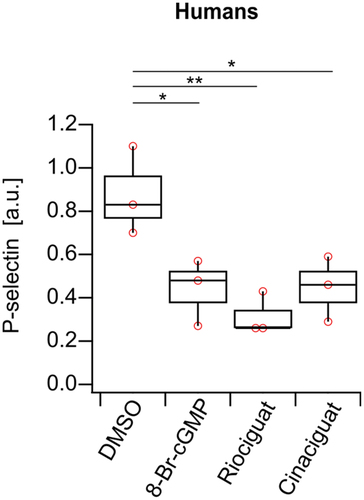
Figure 4. P-selectin signal measured by flow cytometry is decreased in human platelets, when treated with 8-Br-cGMP, riociguat, or cinaciguat. DMSO (1:1000), 8-Br-cGMP (1 mM), riociguat (10 μM), cinaciguat (10 μM). The significance level of P-values is indicated by asterisks (*p < .05; **p < .01; ***p < .001; ns, not significant; Tukey’s test). Experiments were performed with n=3 donors, and each individual point is the average of three samples from the same donor.