Figures & data
Table I. Types of glycosylations involved in cancer metastasis.
Figure 1. Schematic of platelet and surface glycoprotein expression at rest (left, blue-shaded hemisphere) and after activation (right, red-shaded hemisphere). At rest, platelets express a variety of surface glycoproteins, some of which are not activated. The internal membrane structure of platelets is also not activated and almost no content is released. After platelet activation, platelets exhibit a pseudopod-like structure, and some proteins originally located inside the cell appear on the platelet surface; Other proteins are truncated and secreted in soluble form.
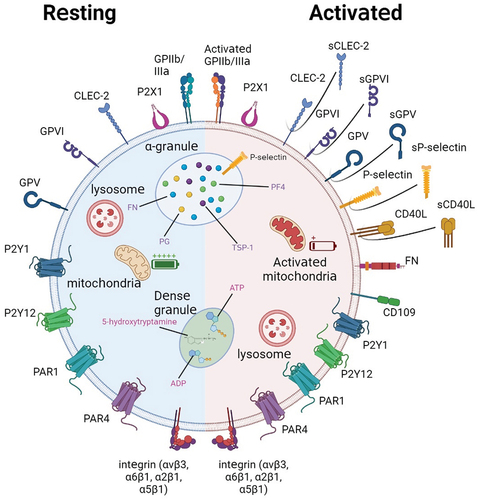
Figure 2. Platelet involvement in cancer metastasis. Once tumor cells enter the bloodstream, a 3-step sequence of events occurs. (1) Platelet-tumor cell aggregates are formed, facilitated through the recognition of multiple glycoproteins. (2) Platelets aggregated on the tumor surface protect CTCs from NK cell-associated cell death. (3) Platelets and their secretion stimulate and help cancer cells to adhere to and penetrate the endothelium, thus supporting cancer cell migration and metastasis formation.
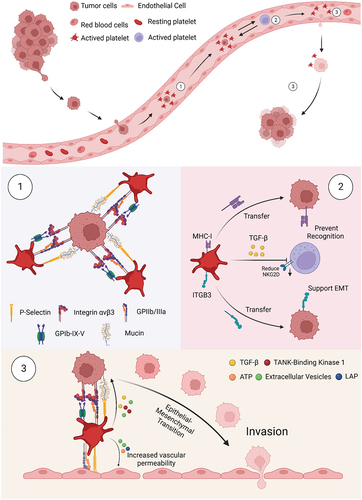
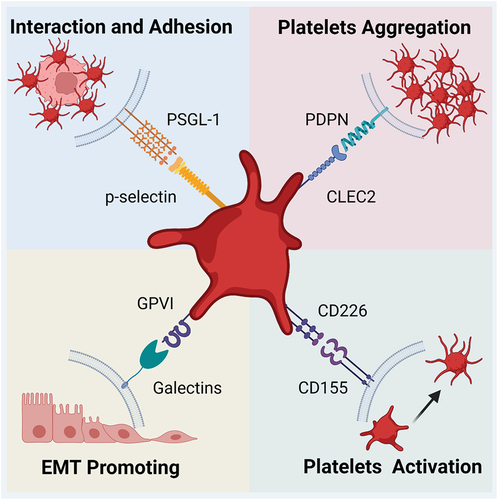
Figure 3. Major glycan-ligand binding present in platelet-associated metastasis. The glycan-lectin binding involves multiple steps from platelet activation to metastasis through the endothelium. The four major lectins include P-selectin, CLEC-2, Galectin, and CD155, and one of their ligands is PSGL-1, PDPN, GPVI, and CD226, respectively. They can affect both platelets and tumor cells. The former may be activated and agglutinated upon glycan-ligand binding, while the latter mainly undergoes adhesion and EMT, ultimately allowing metastasis to be promoted.