Figures & data
Figure 1. Patient enrollment.
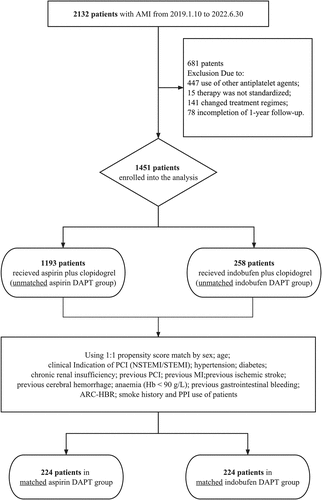
Table I. Clinical characteristics of patients.
Table II. One-year clinical endpoints in patients with AMI.
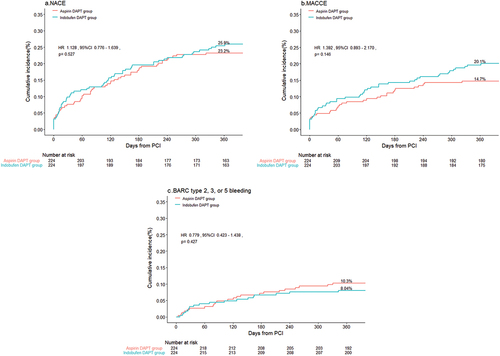
Figure 2. Cumulative Kaplan-Meier curve estimates of NACE (A), MACCE (B), and BARC 2, 3, or 5 (C) at 1 year in patients with AMI.
Supplementary Material
Download ()Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available as the data also forms part of another ongoing study but are available from the corresponding author on reasonable request.