Figures & data
Figure 1. Sera titres of immunised female mice before mating. Alkaline phosphatase conjugated anti-mice IgG and p-nitrophenyl phosphate (PNPP) were used in indirect ELISA, and recombinant unprocessed porcine myostatin was used as a coating antigen.
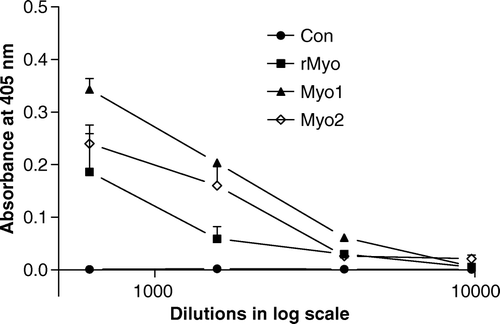
Table 1. Titre values of immunised female mice against three coating antigen.1
Table 2. Changes in body weights of immunised female mice following immunisation against myostatin antigens.
Table 3. Titre values of newborn offspring1
Table 4. Body weights of offspring of the immunised mice up to 8 weeks of age.
Table 5. Carcass, fat and individual muscle weights of offspring at sacrifice at 8 weeks.
Figure 2. Western blot analysis of binding affinity of purified IgG to myostatin. Molecular wt standard (lane 1), 200 ng of mature myostatin (R&D Systems) in non-reduced (lane 2), reduced (lane 3) condition, 4 µg of leg muscle (lane 4) and liver homogenate (lane 5) in reduced condition were fractionated on 15% SDS-PAGE and visualised with Coomassie blue stain (a) or electrophoretically transferred onto a PVDF membrane. The membranes were incubated with 2 µg/ml affinity purified IgG from control (b), rMyo (c), Myo1 (d) and Myo2 (e). Secondary anti-rabbit IgG conjugated to alkaline phosphatase was added at 1:10,000 dilution. Upper and lower arrows indicate myostatin dimer and monomer, respectively. The big protein bands above 50 kDa in lanes 2 and 3 of SDS-PAGE are BSA added as a carrier protein during myostatin preparation.
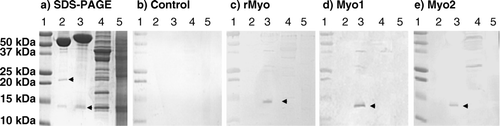
Figure 3. Effects of affinity-purified IgG on myostatin's activity in pGL3 (CAGA)12-luciferase reporter system. A204 cells were seeded (40,000 cells/100 µl/well) on a 96-well culture plate and grown in DMEM with 10% fetal calf serum, antibiotic and antimycotic. After 36 h, a transfection mixture was added containing 0.2 µg of pGL3 (CAGA)12 luc-luciferase plasmid, 0.05 µg of pRL-TK-Renilla luciferase plasmid and 0.5 µl of lipofectamine 2000 in antibiotic-free DMEM containing 10% FCS. After 24 h of transfection, the cells were serum starved for 9 h. After serum starvation, myostatin (5 ng/ml) and various dilutions of the affinity-purified IgGs were added to each well in quadruplets followed by incubation for 6–8 h. Luciferase activity was measured by Veritas microplate luminometer (Turner Biosystems Inc.) using Dual Luciferase Assay System (Promega). Figures A–D represent the ratios of firefly luciferase to renilla luciferase in affinity-purified IgG treated wells from control, rMyo, Myo-1 and Myo-2, respectively. Positive controls were maintained on 5 ng/ml myostatin and 30 ng/ml propeptide while negative controls were on 5 ng/ml myostatin, without propeptide.
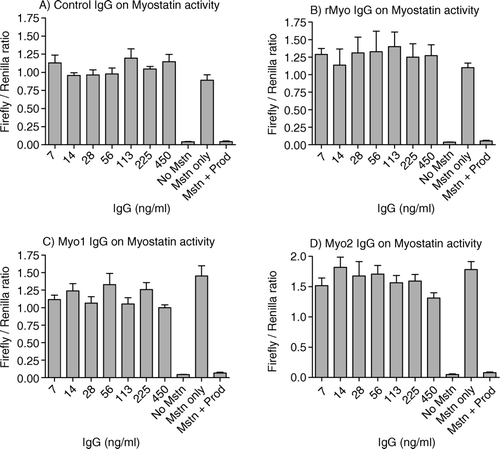
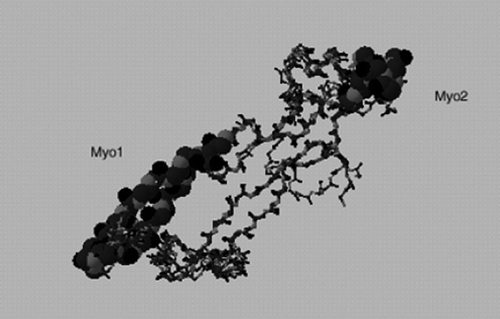
Figure 4. Location of Myo1 and Myo2 peptides used as antigens in predicted 3-D model of myostatin. The model is generated using the SWISS-MODEL Repository (Peitsch, Citation1997).