Figures & data
Figure 1. Effects of FIP-gts and FIP-fve on IFN-γ secretion in PBMCs. (A) Effects of FIP-gts on IFN-γ secretion in hPBMCs. Cultured hPBMCs (2×106 cells/ml, 1 ml/well) were treated with the indicated concentrations of FIP-gts in RPMI 1640 supplemented with 10% FBS for 48 h. Conditioned media were subjected to ELISA to measure amounts of secreted IFN-γ (mean value from three independent experiments). (B) Effects of FIP-fve on IFN-γ secretion in hPBMCs. Cultured peripheral T cells (2×106 cells/ml, 1 ml/well) were treated with the indicated concentrations of FIP-fve in RPMI 1640 supplemented with 10% FBS for 48 h. Conditioned media were subjected to ELISA to measure amounts of secreted IFN-γ (mean value from three independent experiments).
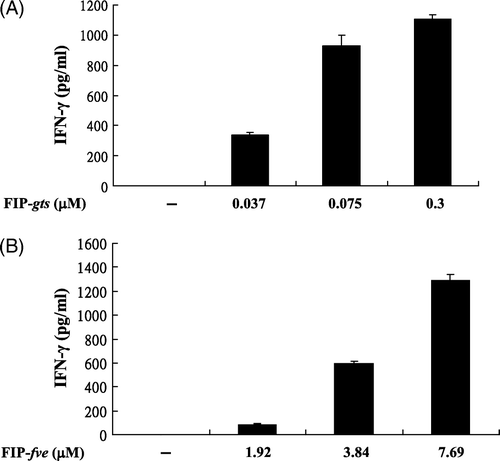
Figure 2. Effect of heating on the stability of FIP-gts and FIP-fve-induced IFN-γ secretion in hPBMCs. The FIP-gts and FIP-fve were pre-incubated at 100°C from 0 to 8 min and residual immunomodulatory activity was determined. Cells cultured without stimulant in the culture supernatants were used as control samples. Human PBMC (2×106 cells/ml) were stimulated by non-heated and heated FIP-gts (0.3 µM) and FIP-fve (7.69 µM) for 48 h. Culture supernatant was collected and assayed for IFN-γ levels by ELISA. The IFN-γ is expressed as pg/ml (mean value from three independent experiments).
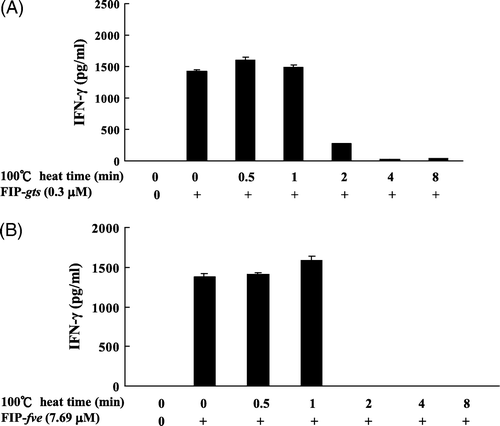
Figure 3. Stability of the deep-frozen and lyophilised FIP-fve with and without additives during storage. The freshly prepared FIP-fve was divided into five groups: (1) only lyophilised; (2) lyophilised with 30% sucrose; (3) lyophilised with 3.2M ammonium sulphate; (4) deep-frozen; and (5) deep-frozen with glycerol. The production of IFN-γ was measured by ELISA. Three independent experiments were performed. Values are means of three independent experiments.
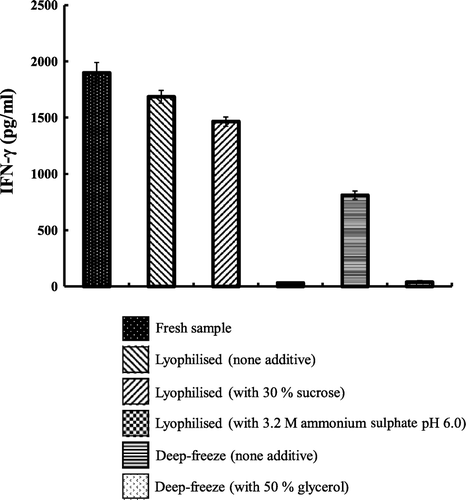
Figure 4. SDS-PAGE analysis of the degradation of FIP-gts and FIP-fve in SGF. (A) FIP-gts and (B) FIP-fve were treated with SGF. Molecular weight markers (lane M) are indicated on the left-hand side of the gel. SGF and the test protein control were run along. The numbers on top denote the incubation times in minutes. The FIP-gts (A) and FIP-fve (B) in SGF digestion were labelled at the right-hand side of the gel, respectively.
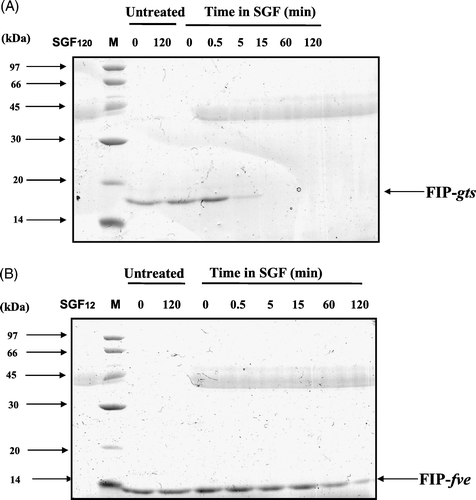
Figure 5. SDS-PAGE analysis of the degradation of FIP-gts and FIP-fve in SIF. (A) FIP-gts and (B) FIP-fve were treated with SIF. Molecular weight markers (lane M) are indicated on the left-hand side of the gel. SIF and the test protein control were run along. The numbers on top denote the incubation times in minutes. The FIP-gts (A) and FIP-fve (B) in SIF digestion were labelled at the right-hand side of the gel.
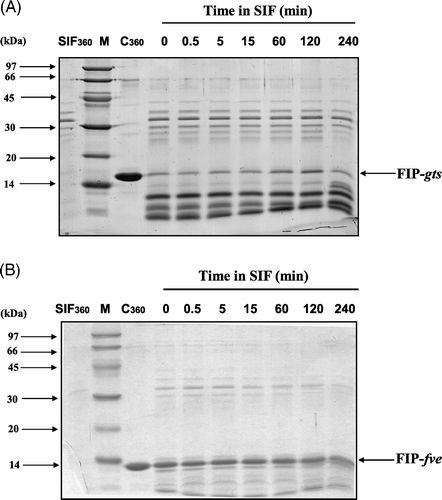
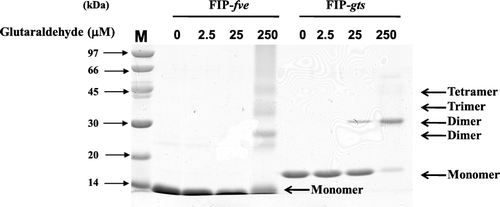
Figure 6. Chemical cross-linking of FIP-gts and FIP-fve. The dimerisation of FIP-gts and FIP-fve were examined by glutaraldehyde cross-linking reaction. About 12 µg of protein was loaded in each lane, and gels were stained with Coomassie blue.