Figures & data
Table 1. Total exposure doses of Cry1Ab in MON810 pollen and leaves per mouse.
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from control group exposed to physiological buffer only.
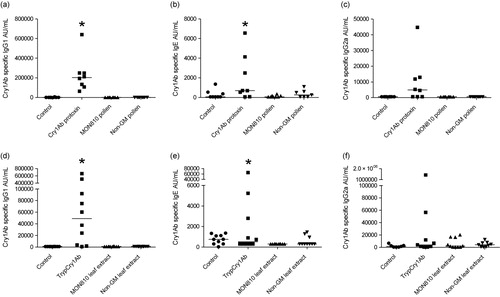
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group (CTRL) exposed to physiological buffer only.
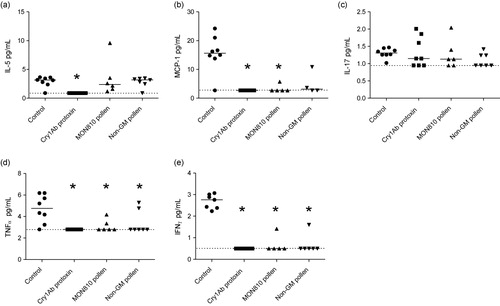
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group exposed to physiological buffer only.
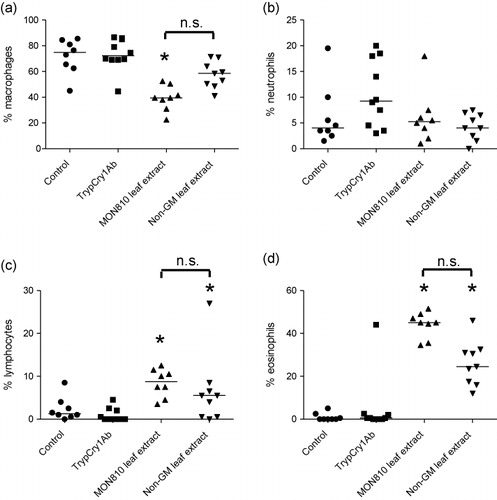
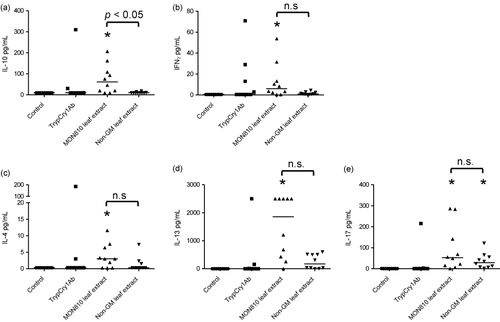
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group exposed to physiological buffer only.