Figures & data
Figure 1. Selection of patients with an HIV diagnosis who initiated ARV therapy involving a minimum of two NRTIs plus one NNRTI or one PI (± ritonavir); ARV, antiretroviral; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
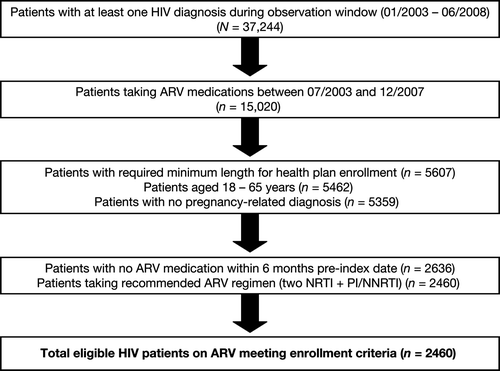
Figure 2. Study schematic. 1The index date occurred between July 2003 and December 2007; 2The ARV regimen involved a minimum of two NRTIs plus one NNRTI or one PI (± ritonavir); 3Nonpersistence was defined as discontinuation of the ARV regimen following an allowed 90-day gap between refills or any change to the initial ARV regimen prescribed; ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
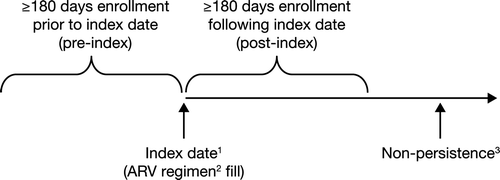
Table 1. Description of regimens.
Table 2. Characteristics of patients (n=2460) included in the study cohort by type of index antiretroviral regimen (NNRTI vs PI).
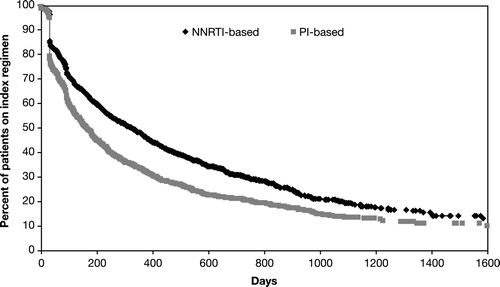
Figure 3. Persistence (i.e., time to discontinuation) by type of index ARV regimen (NNRTI-based or PI-based). The difference in time to discontinuation between PI- and NNRTI-based regimens was p < 0.0001 (log-rank test); ARV, antiretroviral; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.