Figures & data
Figure 1. Participant flow: HIV diagnosis and commencement of antiretroviral therapy (ART).
Note: *23 women with <90 days follow-up reported initiating ART.
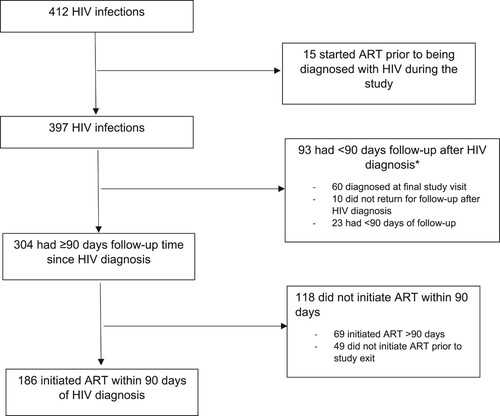
Table 1. Participant characteristics by follow-up status.
Figure 2. Days to antiretroviral therapy (ART) initiation using Kaplan-Meier methods.
Note: *Restricted to women with ≥90 days of follow-up in the trial subsequent to HIV diagnosis.
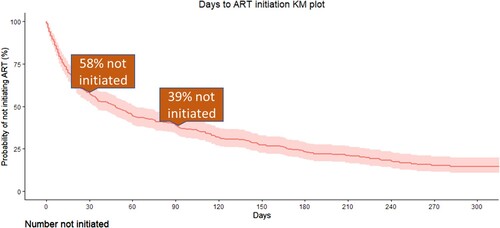
Table 2. Participant characteristics and association with antiretroviral therapy (ART) initiation within 90 days.
Table 3. Reasons women provided for not initiating antiretroviral therapy (ART) at follow-up visits after HIV diagnosis.
Data availability statement
Access to data from the ECHO Trial may be requested through submission of a research concept to [email protected]. The concept must include the research question, data requested, analytic methods, and steps taken to ensure ethical use of the data. Access will be granted if the concept is evaluated to have scientific merit and if sufficient data protections are in place. As of the time of publication, data access applications are in process with the governing institutional review boards of the ECHO Trial to make de-identified data publicly available.
