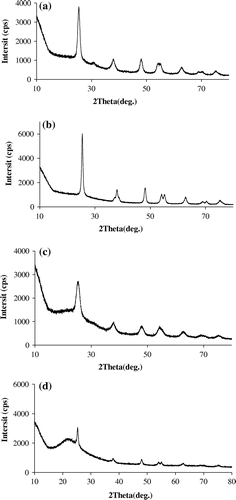
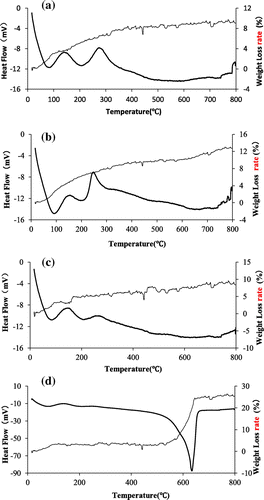
Figures & data
Table 1. Specific surface area of the four TiO2 adsorbents.
Figure 4. Continuous sorption of TiO2(T) (a), TiO2(S) (b), SiO2–TiO2(T) (c), and SiO2–TiO2(S) (d).
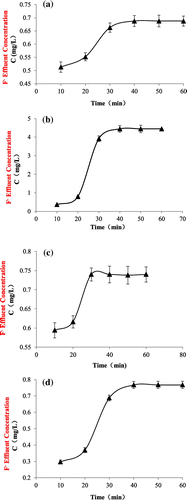
Figure 5. Adsorption capacity (a) and adsorption rate (b) of TiO2(T) and SiO2–TiO2(T) to fluoride with different initial fluoride concentration.
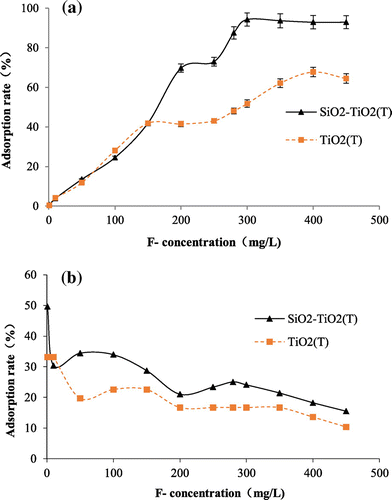
Figure 6. Adsorption capacity (a) and adsorption rate (b) of TiO2(S) and SiO2–TiO2(S) to fluoride with different initial fluoride concentration.
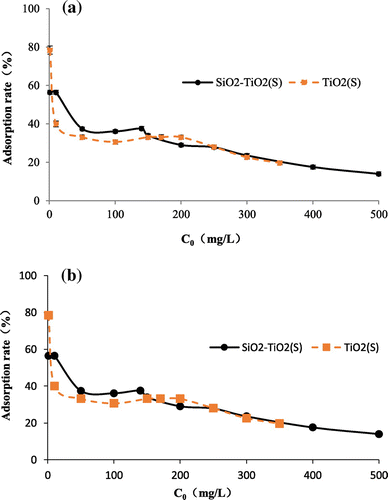
Figure 7. Effect of pH on fluoride adsorption onto TiO2(T), TiO2(S), SiO2–TiO2(T), and SiO2–TiO2(S).
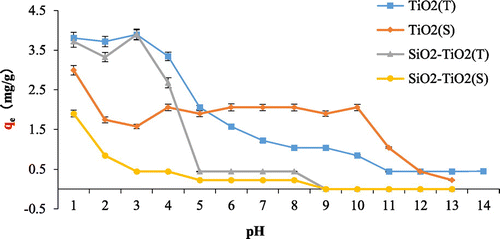
Figure 8. Effects of coexisting ions on fluoride adsorption onto TiO2(T) (a) and TiO2(S) (b).
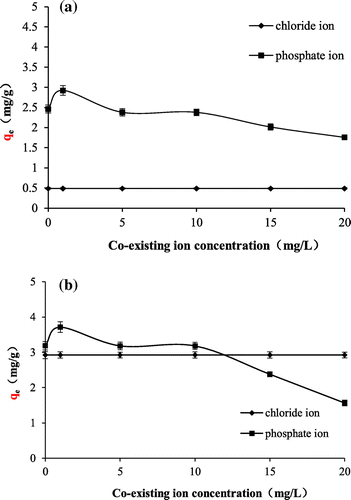
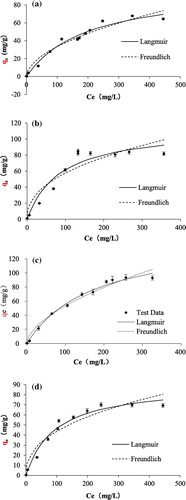
Figure 9. Adsorption isotherms of fluoride on TiO2(T) (a), TiO2(S) (b), SiO2–TiO2(T) (c), and SiO2–TiO2(S) (d).