Figures & data
Table 1. Bulk properties of activated hickory and peanut hull hydrochars.
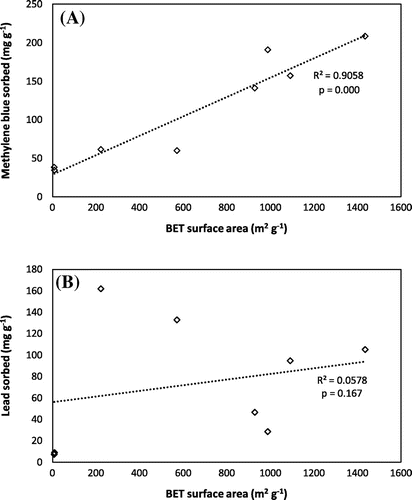
Figure 1. Comparison of chemically AHC with physically AHC and raw HC for the sorption of contaminants. (A) methylene blue and (B) lead.
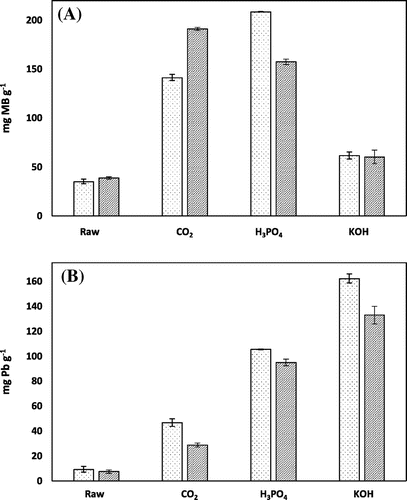
Figure 2. Methylene blue adsorption kinetics: (A) H3PO4 hickory, (B) KOH hickory, (C) H3PO4 peanut, and (D) KOH peanut.
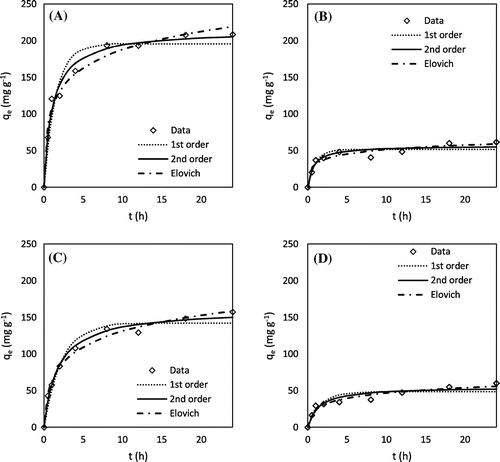
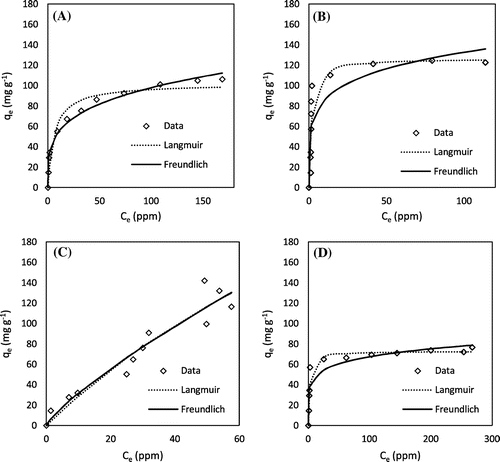
Figure 3. Lead adsorption kinetics: (A) H3PO4 hickory, (B) KOH hickory, (C) H3PO4 peanut, and (D) KOH peanut.
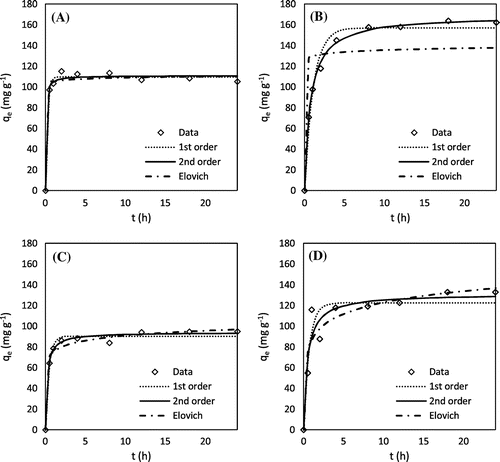
Table 2. Best-fit model parameters of lead sorption on AHCs.
Table 3. Best-fit model parameters of methylene blue sorption on AHCs.
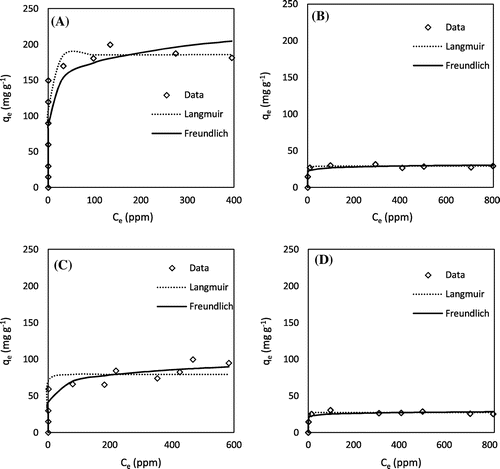
Figure 4. Methylene blue adsorption isotherms: (A) H3PO4 hickory, (B) KOH hickory, (C) H3PO4 peanut, and (D) KOH peanut.