Figures & data
Figure 1. PASI 75, PASI 90, and PASI 100 response at week 12 for patients administered IXEQ2W or ETN by baseline psoriasis severity (NRI). ETN: etanercept; IXEQ2W: ixekizumab 80 mg every 2 weeks; N: total number of patients; NRI: non-responder imputation; PASI: Psoriasis Area and Severity Index. *p < .001, IXEQ2W vs. ETN.
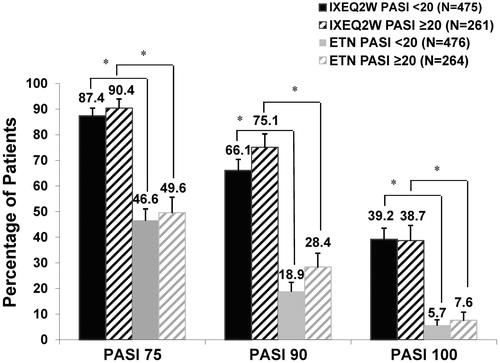
Table 1. Baseline patient demographics and clinical characteristics by baseline psoriasis severity.
Figure 2. Absolute PASI ≤5, PASI ≤2, and PASI ≤1 by baseline psoriasis severity at Week 12 (NRI). ETN: etanercept; IXEQ2W: ixekizumab 80 mg every 2 weeks; N: total number of patients; NRI: non-responder imputation; PASI: Psoriasis Area and Severity Index. *p < .001, IXEQ2W vs. ETN.
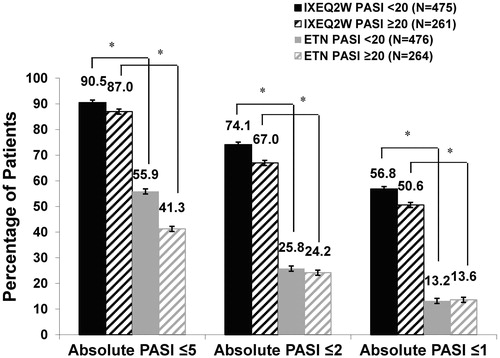
Figure 3. PASI 75, PASI 90, and PASI 100 response by baseline psoriasis severity at Week 156 (mNRI and observed). (A) PASI 75 over 156 weeks of treatment. Some values are obscured where data overlap for the two observed groups. (B) PASI 90 over 156 weeks of treatment. (C) PASI 100 over 156 weeks of treatment. Abbreviations: IXEQ2W: ixekizumab 80 mg every 2 weeks; mNRI: modified non-responder imputation; N: total number of patients; PASI: Psoriasis Area and Severity Index; Q4W: every 4 weeks.
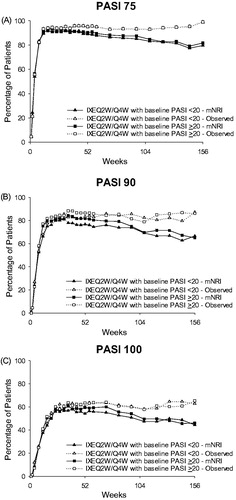
Figure 4. Absolute PASI ≤5, PASI ≤2, and PASI ≤1 by baseline psoriasis severity at Week 156 (mNRI and observed). (A) PASI ≤5 over 156 weeks of treatment. (B) PASI ≤2 over 156 weeks of treatment. (C) PASI ≤1 over 156 weeks of treatment. Abbreviations: IXEQ2W: ixekizumab 80 mg every 2 weeks; mNRI: modified non-responder imputation; N: total number of patients; PASI: Psoriasis Area and Severity Index; Q4W: every 4 weeks.
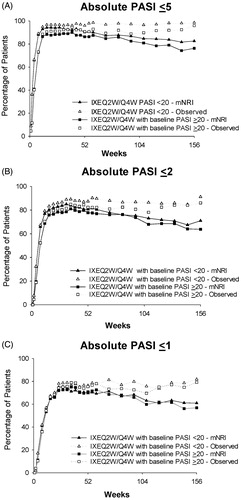
Table 2. Week 12 rates of treatment-emergent adverse events and serious adverse events by baseline psoriasis severity.
Table 3. Week 12–156 rates of treatment-emergent adverse events and serious adverse events by baseline psoriasis severity.
